Journal of Molecular Catalysis A: Chemical Pub Date : 2016-10-24 , DOI: 10.1016/j.molcata.2016.10.027 Yurii A. Borisov , Irena S. Akhrem
|
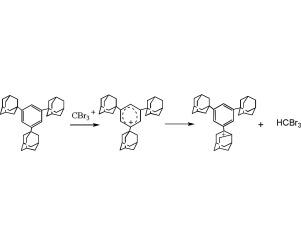
The DFT B3LYP/6-31G* calculations were carried out for the reactions of C6H6 and Ad3C6H3 (Ad = 1,3,5-adamantyl) with superelectrophile CBr3+ as a model of superelectrophilic catalyst CBr3+ Al2Br7−. The reaction of C6H6 with CBr3+ proceeds via the classical scheme of electrophilic reactions of aromatic C − H bond to form initially the barrier-free σ-complex C6H6CBr3+. This mechanism was confirmed by the aug-cc-pVDZ basis set calculations. The reaction of Ad3C6H3 with CBr3+ occurs via a quite novel mechanism involving aryl cation formation followed by hydride abstraction of the Ar+ from the 2-Ad group and the rearrangement of the 2-Ad+ cation into the 4-phenyl-4-protoadamantyl cation. The hydride transfer from both arenes was shown to be more favorable than H radical transfer by more than 40 and 55 kcal mol−1 in the case of C6H6 and Ad3C6H3, respectively.
中文翻译:

С的反应的DFT机理研究6 ħ 6和1,3,5-广告3 Ç 6 ħ 3与CBR 3。氢化物从芳族C
对于C 6 H 6和Ad 3 C 6 H 3(Ad = 1,3,5-金刚烷基)与超亲电试剂CBr 3 +的反应进行了DFT B3LYP / 6-31G *计算,作为超亲电催化剂CBr的模型3 +的Al 2溴7 - 。C的反应6 ħ 6与CBR 3个+前进通过芳族C的亲电反应的经典方案- H键以形成最初的无障碍-σ-络合物C 6 H ^ 6 CBR 3 +。通过aug-cc-pVDZ基集计算确认了该机制。Ad 3 C 6 H 3与CBr 3 +的反应是通过一种非常新颖的机理发生的,该机理涉及芳基阳离子的形成,然后将Ar +的氢化物从2-Ad基团中提取出来,然后将2-Ad +阳离子重排成4个基团。 -苯基-4-原金刚烷基阳离子。在C 6 H 6和Ad 3 C 6 H 3的情况下,从两个芳烃的氢化物转移均比H自由基转移更有利,其转移量大于40和55 kcal mol -1。, 分别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号