Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of mTORC1 in subchondral bone preosteoblasts promotes osteoarthritis by stimulating bone sclerosis and secretion of CXCL12.
Bone Research ( IF 14.3 ) Pub Date : 2019-02-20 , DOI: 10.1038/s41413-018-0041-8 Chuangxin Lin 1, 2 , Liangliang Liu 1 , Chun Zeng 1 , Zhong-Kai Cui 3 , Yuhui Chen 1 , Pinling Lai 1, 3 , Hong Wang 1 , Yan Shao 1 , Haiyan Zhang 1 , Rongkai Zhang 1 , Chang Zhao 1 , Hang Fang 1 , Daozhang Cai 1 , Xiaochun Bai 1, 3
Bone Research ( IF 14.3 ) Pub Date : 2019-02-20 , DOI: 10.1038/s41413-018-0041-8 Chuangxin Lin 1, 2 , Liangliang Liu 1 , Chun Zeng 1 , Zhong-Kai Cui 3 , Yuhui Chen 1 , Pinling Lai 1, 3 , Hong Wang 1 , Yan Shao 1 , Haiyan Zhang 1 , Rongkai Zhang 1 , Chang Zhao 1 , Hang Fang 1 , Daozhang Cai 1 , Xiaochun Bai 1, 3
Affiliation
|
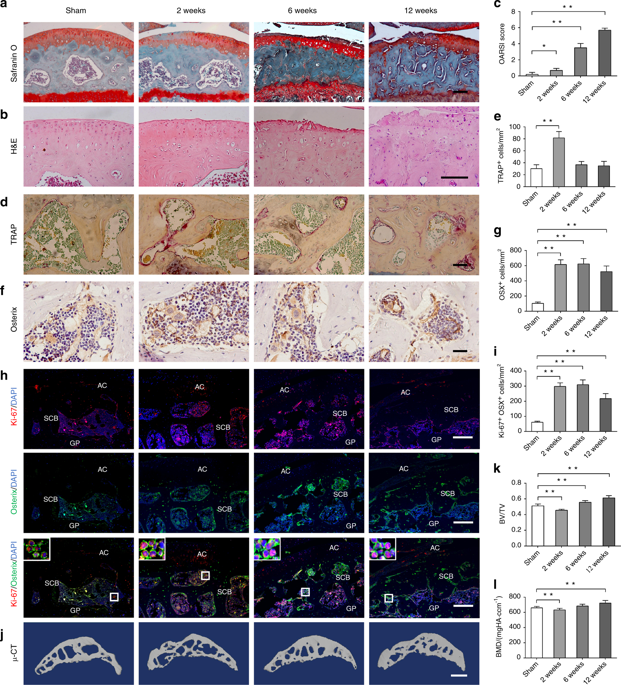
Increasing evidences show that aberrant subchondral bone remodeling plays an important role in the development of osteoarthritis (OA). However, how subchondral bone formation is activated and the mechanism by which increased subchondral bone turnover promotes cartilage degeneration during OA remains unclear. Here, we show that the mechanistic target of rapamycin complex 1 (mTORC1) pathway is activated in subchondral bone preosteoblasts (Osterix+) from OA patients and mice. Constitutive activation of mTORC1 in preosteoblasts by deletion of the mTORC1 upstream inhibitor, tuberous sclerosis 1, induced aberrant subchondral bone formation, and sclerosis with little-to-no effects on articular cartilage integrity, but accelerated post-traumatic OA development in mice. In contrast, inhibition of mTORC1 in preosteoblasts by disruption of Raptor (mTORC1-specific component) reduced subchondral bone formation and cartilage degeneration, and attenuated post-traumatic OA in mice. Mechanistically, mTORC1 activation promoted preosteoblast expansion and Cxcl12 secretion, which induced subchondral bone remodeling and cartilage degeneration during OA. A Cxcl12-neutralizing antibody reduced cartilage degeneration and alleviated OA in mice. Altogether, these findings demonstrate that mTORC1 activation in subchondral preosteoblasts is not sufficient to induce OA, but can induce aberrant subchondral bone formation and secrete of Cxcl12 to accelerate disease progression following surgical destabilization of the joint. Pharmaceutical inhibition of the pathway presents a promising therapeutic approach for OA treatment.
中文翻译:

软骨下骨前成骨细胞中mTORC1的激活通过刺激骨硬化和CXCL12的分泌而促进骨关节炎。
越来越多的证据表明,异常的软骨下骨重塑在骨关节炎(OA)的发展中起着重要作用。然而,如何激活软骨下骨的形成以及软骨下骨更新的增加在OA期间促进软骨变性的机制尚不清楚。在这里,我们显示雷帕霉素复合物1(mTORC1)通路的机械目标在OA患者和小鼠的软骨下骨成骨细胞(Osterix +)中被激活。通过删除mTORC1上游抑制剂,结节性硬化1,诱导软骨下骨异常畸变和硬化,对成骨细胞中的mTORC1进行组成性激活,对关节软骨的完整性几乎没有影响,但加速了小鼠创伤后OA的发展。相比之下,通过破坏猛禽(mTORC1特异性成分)抑制成骨细胞中的mTORC1减少了软骨下骨形成和软骨变性,并减轻了小鼠的创伤后OA。从机制上讲,mTORC1激活可促进成骨细胞的扩增和Cxcl12的分泌,从而诱导OA期间软骨下骨重塑和软骨变性。Cxcl12中和抗体减少了小鼠的软骨变性并减轻了OA。总而言之,这些发现表明软骨下成骨细胞中的mTORC1活化不足以诱导OA,但可以诱导异常的软骨下骨形成和Cxcl12分泌,从而加速关节手术不稳定后的疾病进展。对该途径的药物抑制为OA治疗提供了一种有前途的治疗方法。
更新日期:2019-02-20
中文翻译:

软骨下骨前成骨细胞中mTORC1的激活通过刺激骨硬化和CXCL12的分泌而促进骨关节炎。
越来越多的证据表明,异常的软骨下骨重塑在骨关节炎(OA)的发展中起着重要作用。然而,如何激活软骨下骨的形成以及软骨下骨更新的增加在OA期间促进软骨变性的机制尚不清楚。在这里,我们显示雷帕霉素复合物1(mTORC1)通路的机械目标在OA患者和小鼠的软骨下骨成骨细胞(Osterix +)中被激活。通过删除mTORC1上游抑制剂,结节性硬化1,诱导软骨下骨异常畸变和硬化,对成骨细胞中的mTORC1进行组成性激活,对关节软骨的完整性几乎没有影响,但加速了小鼠创伤后OA的发展。相比之下,通过破坏猛禽(mTORC1特异性成分)抑制成骨细胞中的mTORC1减少了软骨下骨形成和软骨变性,并减轻了小鼠的创伤后OA。从机制上讲,mTORC1激活可促进成骨细胞的扩增和Cxcl12的分泌,从而诱导OA期间软骨下骨重塑和软骨变性。Cxcl12中和抗体减少了小鼠的软骨变性并减轻了OA。总而言之,这些发现表明软骨下成骨细胞中的mTORC1活化不足以诱导OA,但可以诱导异常的软骨下骨形成和Cxcl12分泌,从而加速关节手术不稳定后的疾病进展。对该途径的药物抑制为OA治疗提供了一种有前途的治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号