当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Critical design factors for kinetically favorable P-based compounds toward alloying with Na ions for high-power sodium-ion batteries†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2019-02-15 00:00:00 , DOI: 10.1039/c9ee00283a Duho Kim 1, 2, 3, 4 , Kai Zhang 4, 5, 6, 7 , Maenghyo Cho 4, 8, 9, 10 , Yong-Mook Kang 4, 5, 6, 7
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2019-02-15 00:00:00 , DOI: 10.1039/c9ee00283a Duho Kim 1, 2, 3, 4 , Kai Zhang 4, 5, 6, 7 , Maenghyo Cho 4, 8, 9, 10 , Yong-Mook Kang 4, 5, 6, 7
Affiliation
|
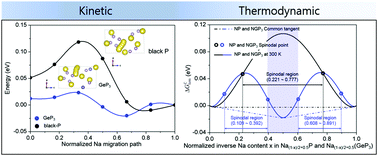
First-principles calculations with experimental validation enable us to radically predict and compare the electron transport, elastic softness, and thermodynamic phase stabilities of black phosphorus (BP) and GeP3 to figure out the crucial factors for designing the most kinetically favorable P-based compounds toward alloying with Na ions. From the viewpoint of electron transport, Ge is able to provide P with electrons due to the difference of electronegativity, causing GeP3 to have a significantly reduced band gap and a lower electron-hopping barrier compared to BP. Because the structure of GeP3 looks more elastically softened than that of BP, its Na+ migration barriers in the sodiated states must be lower than those of the sodiated BP structure as proven by the higher Na+ diffusion coefficients of GeP3. The thermodynamic phase stabilities of BP and GeP3 could be predicted by calculating their mixing enthalpy values and thereby homogeneous bulk free energies were obtained for Na1−x(GeP3) (0.0 ≤ x ≤ 0.5) indicating that no phase separation occurs in the sodiated GeP3 because of an additional stable single-phase at x = 0.25. By contrast, the sodiated P easily gets separated into NaP and Na0.5P with the same sodiation content. The suppressed phase separation in GeP3 makes the Na+ distribution homogeneous as confirmed by TEM-EDS color mapping and thus contributes to increasing Na+ diffusion coefficients because Na+ migration can be primarily interrupted by the interfaces or grain boundaries between the separated phases. Taking into account three enhanced physicochemical properties, the GeP3 electrodes may well present a superior rate capability to the BP electrode, underscoring that electron transport, elastic softness, and thermodynamic phase stabilities must be the most important design factors for realizing kinetically favorable P-based compounds toward alloying with Na+. In detail, even at an ultrahigh current density of 20 A g−1, the capacity of the GeP3 electrode still comes to 0.197 A h g−1. At 1 A g−1, GeP3 could maintain a discharge capacity of 0.499 A h g−1 even after 1000 cycles. This study may provide promising guidelines for the design of alloy-based electrode materials for high power and energy SIBs.
中文翻译:

大功率钠离子电池中具有动力学优势的P基化合物与Na离子合金化的关键设计因素†
通过第一性原理计算和实验验证,我们能够从根本上预测和比较黑磷(BP)和GeP 3的电子传递,弹性软度和热力学相稳定性,从而找出设计最具有动力学优势的P基化合物的关键因素与Na离子合金化。从电子传输的角度来看,由于电负性的差异,Ge能够为P提供电子,与BP相比,GeP 3的带隙显着减小,电子跃迁势垒更低。由于GeP 3的结构看起来比BP的弹性更软,因此它的Na +如GeP 3的较高的Na +扩散系数所证明的,在固态状态下的迁移势垒必须低于在BP结构下的迁移势垒。BP和的GEP的热力学相稳定性3可以通过计算它们的混合焓值,从而均匀散装自由能的Na获得预测1- X(GEP 3)(0.0≤ X ≤0.5),这表明没有相分离的发生因为在x = 0.25时有一个额外的稳定单相,所以生成了GeP 3。相比之下,纯净的P容易分离为NaP和Na 0.5P具有相同的添加量。GeP 3中抑制的相分离使TEM-EDS色彩映射所证实的Na +分布均匀,因此有助于提高Na +扩散系数,因为Na +迁移主要被分离相之间的界面或晶界中断。考虑到三种增强的理化特性,GeP 3电极可能具有比BP电极更高的倍率能力,强调电子传输,弹性柔软度和热力学相稳定性必须是实现动力学上有利的P基电极的最重要设计因素。化合物与Na +合金化。详细地,即使在20A g -1的超高电流密度下,GeP 3电极的容量仍然达到0.197 A hg -1。在1 A g -1下,即使经过1000次循环,GeP 3仍可保持0.499 A hg -1的放电容量。这项研究可能为高功率和高能量SIB的合金基电极材料的设计提供有希望的指导。
更新日期:2019-02-15
中文翻译:

大功率钠离子电池中具有动力学优势的P基化合物与Na离子合金化的关键设计因素†
通过第一性原理计算和实验验证,我们能够从根本上预测和比较黑磷(BP)和GeP 3的电子传递,弹性软度和热力学相稳定性,从而找出设计最具有动力学优势的P基化合物的关键因素与Na离子合金化。从电子传输的角度来看,由于电负性的差异,Ge能够为P提供电子,与BP相比,GeP 3的带隙显着减小,电子跃迁势垒更低。由于GeP 3的结构看起来比BP的弹性更软,因此它的Na +如GeP 3的较高的Na +扩散系数所证明的,在固态状态下的迁移势垒必须低于在BP结构下的迁移势垒。BP和的GEP的热力学相稳定性3可以通过计算它们的混合焓值,从而均匀散装自由能的Na获得预测1- X(GEP 3)(0.0≤ X ≤0.5),这表明没有相分离的发生因为在x = 0.25时有一个额外的稳定单相,所以生成了GeP 3。相比之下,纯净的P容易分离为NaP和Na 0.5P具有相同的添加量。GeP 3中抑制的相分离使TEM-EDS色彩映射所证实的Na +分布均匀,因此有助于提高Na +扩散系数,因为Na +迁移主要被分离相之间的界面或晶界中断。考虑到三种增强的理化特性,GeP 3电极可能具有比BP电极更高的倍率能力,强调电子传输,弹性柔软度和热力学相稳定性必须是实现动力学上有利的P基电极的最重要设计因素。化合物与Na +合金化。详细地,即使在20A g -1的超高电流密度下,GeP 3电极的容量仍然达到0.197 A hg -1。在1 A g -1下,即使经过1000次循环,GeP 3仍可保持0.499 A hg -1的放电容量。这项研究可能为高功率和高能量SIB的合金基电极材料的设计提供有希望的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号