当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-catalysed asymmetric dearomative formal [4+2] cycloadditions of 3-benzofuranyl vinyl ketones†
Chemical Communications ( IF 4.3 ) Pub Date : 2019-02-15 00:00:00 , DOI: 10.1039/c9cc00386j Ben-Xian Xiao 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5 , Xue Song 1, 2, 3, 4, 5 , Wei Du 1, 2, 3, 4, 5 , Ying-Chun Chen 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2019-02-15 00:00:00 , DOI: 10.1039/c9cc00386j Ben-Xian Xiao 1, 2, 3, 4, 5 , Bo Jiang 1, 2, 3, 4, 5 , Xue Song 1, 2, 3, 4, 5 , Wei Du 1, 2, 3, 4, 5 , Ying-Chun Chen 1, 2, 3, 4, 5
Affiliation
|
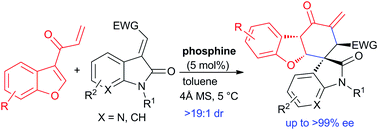
In contrast to the catalytic asymmetric dearomative reactions of indole substrates, those of the analogous benzofuran derivatives have been less explored. Here we report that the stereoselective domino Rauhut–Currier/Michael addition process of 3-benzofuranyl vinyl ketones and 3-olefinic (7-aza)oxindoles can be realised via catalysis by a chiral bifunctional phosphine, furnishing the previously unreported direct asymmetric dearomative reaction of benzofuran substrates tethered to a carbonyl group in a formal [4+2] cycloaddition manner. An array of hydrodibenzofuran derivatives with dense substitutions is generally constructed with excellent diastereoselectivity and enantioselectivity (up to >19 : 1 dr, >99% ee).
中文翻译:

膦催化的3-苯并呋喃基乙烯基酮的不对称脱芳香性正[4 + 2]环加成反应†
与吲哚底物的催化不对称脱芳香反应相反,类似苯并呋喃衍生物的反应很少被研究。在这里我们报告说,3-苯并呋喃基乙烯基酮和3-烯烃(7-氮杂)恶吲哚的立体选择性多米诺骨牌的Rauhut-Currier / Michael加成过程可以通过手性双官能膦催化来实现,从而提供了以前未报道的直接的不对称脱芳香反应。苯并呋喃底物以正式的[4 + 2]环加成方式束缚在羰基上。通常以优异的非对映选择性和对映选择性(高达> 19:1 dr,> 99%ee)构建一系列具有高取代度的氢二苯并呋喃衍生物。
更新日期:2019-02-15
中文翻译:

膦催化的3-苯并呋喃基乙烯基酮的不对称脱芳香性正[4 + 2]环加成反应†
与吲哚底物的催化不对称脱芳香反应相反,类似苯并呋喃衍生物的反应很少被研究。在这里我们报告说,3-苯并呋喃基乙烯基酮和3-烯烃(7-氮杂)恶吲哚的立体选择性多米诺骨牌的Rauhut-Currier / Michael加成过程可以通过手性双官能膦催化来实现,从而提供了以前未报道的直接的不对称脱芳香反应。苯并呋喃底物以正式的[4 + 2]环加成方式束缚在羰基上。通常以优异的非对映选择性和对映选择性(高达> 19:1 dr,> 99%ee)构建一系列具有高取代度的氢二苯并呋喃衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号