当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modular genetic design of multi-domain functional amyloids: insights into self-assembly and functional properties†
Chemical Science ( IF 7.6 ) Pub Date : 2019-02-15 00:00:00 , DOI: 10.1039/c9sc00208a Mengkui Cui 1, 2, 3 , Qi Qi 1 , Thomas Gurry 4 , Tianxin Zhao 1, 2, 3 , Bolin An 1 , Jiahua Pu 1 , Xinrui Gui 3, 5 , Allen A Cheng 6 , Siyu Zhang 1 , Dongmin Xun 1 , Michele Becce 6, 7, 8 , Francesco Briatico-Vangosa 7 , Cong Liu 5 , Timothy K Lu 6 , Chao Zhong 1
Chemical Science ( IF 7.6 ) Pub Date : 2019-02-15 00:00:00 , DOI: 10.1039/c9sc00208a Mengkui Cui 1, 2, 3 , Qi Qi 1 , Thomas Gurry 4 , Tianxin Zhao 1, 2, 3 , Bolin An 1 , Jiahua Pu 1 , Xinrui Gui 3, 5 , Allen A Cheng 6 , Siyu Zhang 1 , Dongmin Xun 1 , Michele Becce 6, 7, 8 , Francesco Briatico-Vangosa 7 , Cong Liu 5 , Timothy K Lu 6 , Chao Zhong 1
Affiliation
|
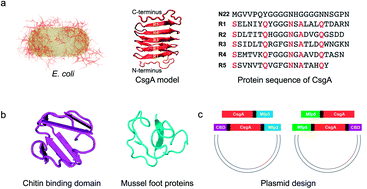
Engineering functional amyloids through a modular genetic strategy represents new opportunities for creating multifunctional molecular materials with tailored structures and performance. Despite important advances, how fusion modules affect the self-assembly and functional properties of amyloids remains elusive. Here, using Escherichia coli curli as a model system, we systematically studied the effect of flanking domains on the structures, assembly kinetics and functions of amyloids. The designed amyloids were composed of E. coli biofilm protein CsgA (as amyloidogenic cores) and one or two flanking domains, consisting of chitin-binding domains (CBDs) from Bacillus circulans chitinase, and/or mussel foot proteins (Mfps). Incorporation of fusion domains did not disrupt the typical β-sheet structures, but indeed affected assembly rate, morphology, and stiffness of resultant fibrils. Consequently, the CsgA-fusion fibrils, particularly those containing three domains, were much shorter than the CsgA-only fibrils. Furthermore, the stiffness of the resultant fibrils was heavily affected by the structural feature of fusion domains, with β-sheet-containing domains tending to increase the Young's modulus while random coil domains decreasing the Young's modulus. In addition, fibrils containing CBD domains showed higher chitin-binding activity compared to their CBD-free counterparts. The CBD-CsgA-Mfp3 construct exhibited significantly lower binding activity than Mfp5-CsgA-CBD due to inappropriate folding of the CBD domain in the former construct, in agreement with results based upon molecular dynamics modeling. Our study provides new insights into the assembly and functional properties of designer amyloid proteins with increasing complex domain structures and lays the foundation for the future design of functional amyloid-based structures and molecular materials.
中文翻译:

多域功能性淀粉样蛋白的模块化遗传设计:对自组装和功能特性的见解†
通过模块化遗传策略工程化功能性淀粉样蛋白为创造具有定制结构和性能的多功能分子材料提供了新的机会。尽管取得了重要进展,但融合模块如何影响淀粉样蛋白的自组装和功能特性仍然难以捉摸。在这里,我们以大肠杆菌curli为模型系统,系统地研究了侧翼结构域对淀粉样蛋白的结构、组装动力学和功能的影响。设计的淀粉样蛋白由大肠杆菌生物膜蛋白 CsgA(作为淀粉样蛋白生成核心)和一个或两个侧翼结构域组成,该侧翼结构域由来自环状芽孢杆菌几丁质酶的几丁质结合结构域 (CBD) 和/或贻贝足蛋白 (Mfps) 组成。融合结构域的合并并没有破坏典型的β-折叠结构,但确实影响了所得原纤维的组装速率、形态和刚度。因此,CsgA 融合原纤维,特别是那些包含三个结构域的原纤维,比仅 CsgA 的原纤维短得多。此外,所得原纤维的刚度受到融合域结构特征的严重影响,含有β-折叠的域往往会增加杨氏模量,而无规卷曲域会降低杨氏模量。此外,与不含 CBD 的原纤维相比,含有 CBD 结构域的原纤维表现出更高的几丁质结合活性。 CBD-CsgA-Mfp3 构建体表现出比 Mfp5-CsgA-CBD 显着更低的结合活性,这是由于前一构建体中 CBD 结构域的不适当折叠,这与基于分子动力学模型的结果一致。 我们的研究为具有日益复杂的结构域结构的设计淀粉样蛋白的组装和功能特性提供了新的见解,并为未来基于淀粉样蛋白的功能性结构和分子材料的设计奠定了基础。
更新日期:2019-02-15
中文翻译:

多域功能性淀粉样蛋白的模块化遗传设计:对自组装和功能特性的见解†
通过模块化遗传策略工程化功能性淀粉样蛋白为创造具有定制结构和性能的多功能分子材料提供了新的机会。尽管取得了重要进展,但融合模块如何影响淀粉样蛋白的自组装和功能特性仍然难以捉摸。在这里,我们以大肠杆菌curli为模型系统,系统地研究了侧翼结构域对淀粉样蛋白的结构、组装动力学和功能的影响。设计的淀粉样蛋白由大肠杆菌生物膜蛋白 CsgA(作为淀粉样蛋白生成核心)和一个或两个侧翼结构域组成,该侧翼结构域由来自环状芽孢杆菌几丁质酶的几丁质结合结构域 (CBD) 和/或贻贝足蛋白 (Mfps) 组成。融合结构域的合并并没有破坏典型的β-折叠结构,但确实影响了所得原纤维的组装速率、形态和刚度。因此,CsgA 融合原纤维,特别是那些包含三个结构域的原纤维,比仅 CsgA 的原纤维短得多。此外,所得原纤维的刚度受到融合域结构特征的严重影响,含有β-折叠的域往往会增加杨氏模量,而无规卷曲域会降低杨氏模量。此外,与不含 CBD 的原纤维相比,含有 CBD 结构域的原纤维表现出更高的几丁质结合活性。 CBD-CsgA-Mfp3 构建体表现出比 Mfp5-CsgA-CBD 显着更低的结合活性,这是由于前一构建体中 CBD 结构域的不适当折叠,这与基于分子动力学模型的结果一致。 我们的研究为具有日益复杂的结构域结构的设计淀粉样蛋白的组装和功能特性提供了新的见解,并为未来基于淀粉样蛋白的功能性结构和分子材料的设计奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号