Chemical Physics Letters ( IF 2.8 ) Pub Date : 2019-02-13 , DOI: 10.1016/j.cplett.2019.02.010 Manjunath D. Meti , Mayank Dixit , Timir Hajari , B.L. Tembe
|
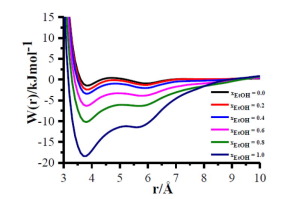
Constrained molecular dynamics simulations are used to compute the potentials of mean force (PMFs) of 1-butyl-3-methylimidazolium chloride (BMIM+−Cl−) ion pair in water-ethanol mixtures. From the PMFs of BMIM+−Cl− ion pair, we notice that, as the mole fraction of ethanol increases, the depths of the minima of the contact ion pair (CIP) and solvent assisted ion pair (SAIP) increase. The CIPs and SAIPs are stabilized by entropy in all the mixtures. As the mole fraction of ethanol changes from 1.0 to 0.8, the stabilities of CIP and SAIP are reduced significantly due to large enhancement in the local densities of water.
中文翻译:

分子动力学模拟的水-乙醇混合物中丁基甲基咪唑鎓氯化物离子对的离子配对和优先溶剂化
约束分子动力学模拟来计算的平均力电位的1-丁基-3-甲基咪唑氯化物(BMIM(保偏光纤)+ -Cl - )在水-乙醇混合物的离子对。从BMIM的的PMF + -Cl -离子对,我们注意到的是,由于增加乙醇的摩尔分数,所述接触离子对(CIP)和溶剂辅助离子对(SAIP)增加的最小值的深度。CIP和SAIP通过所有混合物中的熵来稳定。当乙醇的摩尔分数从1.0变为0.8时,由于水的局部密度大大提高,CIP和SAIP的稳定性大大降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号