Chemical Physics Letters ( IF 2.8 ) Pub Date : 2019-02-11 , DOI: 10.1016/j.cplett.2019.01.053 Cornelie Bänsch , Matthias Olzmann
|
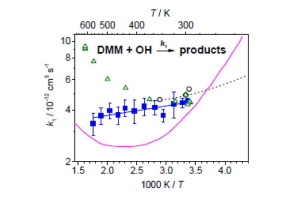
The rate coefficient of the reaction of dimethoxymethane with OH radicals was determined in a slow-flow reactor with pulsed laser photolysis/laser-induced fluorescence technique. Temperatures ranged from 297–570 K at nominal pressures of 2, 5, and 10 bar (bath gas: argon). The very weak temperature dependence of the obtained rate coefficient can be expressed as: k1(T) = (2.9 ± 0.2) × 10–12 exp[(126 ± 30)/T] cm3 s–1. A significant pressure dependence was not observed. The results are in very good agreement with literature data for temperatures up to 370 K whereas differences occur at T > 400 K.
中文翻译:

二甲氧基甲烷与羟基自由基的反应:在高于296 K的温度,2、5和10 bar的压力下进行的实验动力学研究
用脉冲激光光解/激光诱导荧光技术在慢流反应器中确定二甲氧基甲烷与OH自由基的反应速率系数。在标称压力为2、5和10 bar(浴气体:氩气)下,温度范围为297–570K。所得速率系数的非常弱的温度依赖性可以表示为:k 1(T)=(2.9±0.2)×10 –12 exp [(126±30)/ T ] cm 3 s –1。没有观察到明显的压力依赖性。该结果与温度高达370 K的文献数据非常吻合,而在T > 400 K时会出现差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号