当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Hydrogenation Performance of RuMo Phosphide for Chemoselective Reduction of Functionalized Aromatic Hydrocarbons
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-02-21 , DOI: 10.1021/acs.iecr.8b06295 Yolanda Bonita 1 , Timothy P. O’Connell 1 , Holly E. Miller 1 , Jason C. Hicks 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-02-21 , DOI: 10.1021/acs.iecr.8b06295 Yolanda Bonita 1 , Timothy P. O’Connell 1 , Holly E. Miller 1 , Jason C. Hicks 1
Affiliation
|
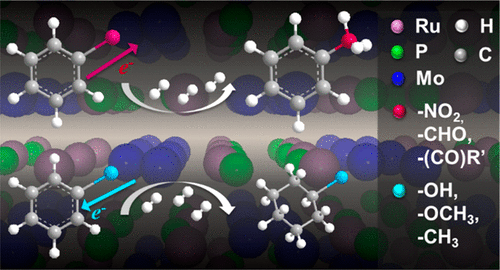
Bimetallic transition metal phosphide catalysts are promising materials for low-temperature, liquid-phase hydrogenation reactions. This work explores the chemoselective hydrogenation ability of RuMoP using various functionalized aromatic hydrocarbons to provide insight into how the functional groups compete for reduction on the surface of RuMoP. Using molecular hydrogen as the reductant, high selectivity (∼99%) to reduction of the substituent is achieved for the hydrogenation of electron withdrawing functionalities such as nitrobenzene, benzaldehyde, and benzophenone with RuMoP to yield aniline, benzyl alcohol, and diphenylmethanol, respectively. In contrast, aromatics with electron donating groups such as phenol, anisole, and toluene, show high ring hydrogenation selectivity (∼99%) to form cyclohexanol, methoxycyclohexane, and methyl cyclohexane, respectively, although the reaction proceeded slowly with RuMoP. Pyridine adsorption was studied via diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), which provided evidence of surface electron deficient sites (i.e., Lewis acids) that are responsible for targeting the electron-rich portion of the substrate. Additional DRIFTS experiments were performed using nitrobenzene, anisole, and a mixture of the two. From these experiments, features associated with −NO2 adsorption in nitrobenzene and ring adsorption in anisole were observed, which correlated well with the observed reaction results. Finally, a solvent study provided evidence for the competitive adsorption of isopropanol and the π-electrons from the aromatic ring of phenol with the former being more favorable on RuMoP surface.
中文翻译:

揭示RuMo磷化物的加氢性能,用于化学选择性还原官能化芳烃
双金属过渡金属磷化物催化剂是用于低温液相加氢反应的有前途的材料。这项工作探索了使用各种官能化的芳香烃对RuMoP进行化学选择性加氢的能力,从而洞察了这些官能团如何竞争RuMoP表面的还原。使用分子氢作为还原剂,通过RuMoP氢化硝基苯,苯甲醛和二苯甲酮等吸电子官能团可分别获得苯胺,苯甲醇和二苯甲醇,从而对取代基的还原反应具有很高的选择性(〜99%)。相反,带有给电子基团的芳族化合物(例如苯酚,苯甲醚和甲苯)显示出较高的环氢化选择性(〜99%),形成环己醇,甲氧基环己烷和甲基环己烷,分别使用RuMoP进行反应,尽管反应进行缓慢。通过漫反射红外傅里叶变换光谱法(DRIFTS)研究了吡啶的吸附,该法提供了表面电子不足位点(即路易斯酸)的证据,这些位点负责靶向底物的富电子部分。使用硝基苯,苯甲醚和两者的混合物进行了另外的DRIFTS实验。从这些实验中,与−NO相关的特征 使用硝基苯,苯甲醚和两者的混合物进行了另外的DRIFTS实验。从这些实验中,与−NO相关的特征 使用硝基苯,苯甲醚和两者的混合物进行了另外的DRIFTS实验。从这些实验中,与−NO相关的特征观察到2在硝基苯中的吸附和在苯甲醚中的环吸附,这与观察到的反应结果密切相关。最后,溶剂研究为异丙醇和苯酚芳环的π电子竞争性吸附提供了证据,前者在RuMoP表面更有利。
更新日期:2019-02-21
中文翻译:

揭示RuMo磷化物的加氢性能,用于化学选择性还原官能化芳烃
双金属过渡金属磷化物催化剂是用于低温液相加氢反应的有前途的材料。这项工作探索了使用各种官能化的芳香烃对RuMoP进行化学选择性加氢的能力,从而洞察了这些官能团如何竞争RuMoP表面的还原。使用分子氢作为还原剂,通过RuMoP氢化硝基苯,苯甲醛和二苯甲酮等吸电子官能团可分别获得苯胺,苯甲醇和二苯甲醇,从而对取代基的还原反应具有很高的选择性(〜99%)。相反,带有给电子基团的芳族化合物(例如苯酚,苯甲醚和甲苯)显示出较高的环氢化选择性(〜99%),形成环己醇,甲氧基环己烷和甲基环己烷,分别使用RuMoP进行反应,尽管反应进行缓慢。通过漫反射红外傅里叶变换光谱法(DRIFTS)研究了吡啶的吸附,该法提供了表面电子不足位点(即路易斯酸)的证据,这些位点负责靶向底物的富电子部分。使用硝基苯,苯甲醚和两者的混合物进行了另外的DRIFTS实验。从这些实验中,与−NO相关的特征 使用硝基苯,苯甲醚和两者的混合物进行了另外的DRIFTS实验。从这些实验中,与−NO相关的特征 使用硝基苯,苯甲醚和两者的混合物进行了另外的DRIFTS实验。从这些实验中,与−NO相关的特征观察到2在硝基苯中的吸附和在苯甲醚中的环吸附,这与观察到的反应结果密切相关。最后,溶剂研究为异丙醇和苯酚芳环的π电子竞争性吸附提供了证据,前者在RuMoP表面更有利。











































 京公网安备 11010802027423号
京公网安备 11010802027423号