Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The induction of core pluripotency master regulators in cancers defines poor clinical outcomes and treatment resistance.
Oncogene ( IF 6.9 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41388-019-0712-y A C Hepburn 1 , R E Steele 2 , R Veeratterapillay 3 , L Wilson 1 , E E Kounatidou 1 , A Barnard 1 , P Berry 1 , J R Cassidy 1 , M Moad 1 , A El-Sherif 4 , L Gaughan 1 , I G Mills 2, 5 , C N Robson 1 , R Heer 1, 3
Oncogene ( IF 6.9 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41388-019-0712-y A C Hepburn 1 , R E Steele 2 , R Veeratterapillay 3 , L Wilson 1 , E E Kounatidou 1 , A Barnard 1 , P Berry 1 , J R Cassidy 1 , M Moad 1 , A El-Sherif 4 , L Gaughan 1 , I G Mills 2, 5 , C N Robson 1 , R Heer 1, 3
Affiliation
|
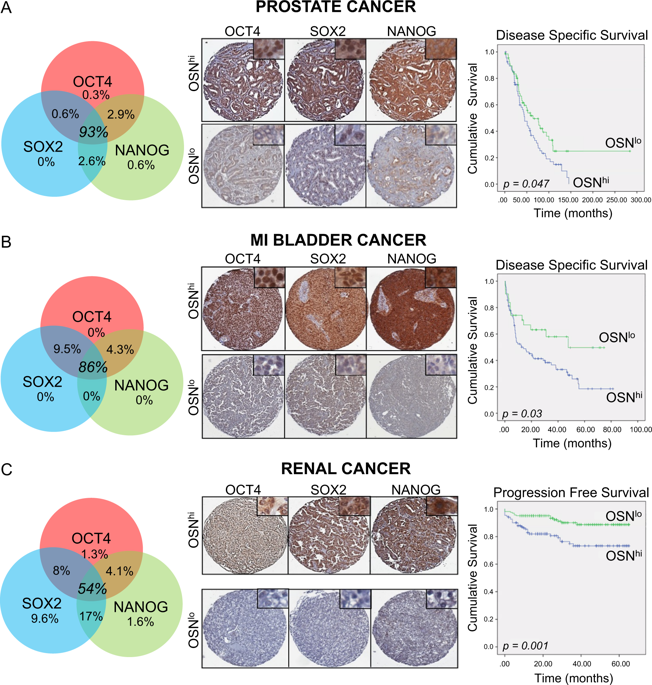
Stem cell characteristics have been associated with treatment resistance and poor prognosis across many cancer types. The ability to induce and regulate the pathways that sustain these characteristic hallmarks of lethal cancers in a novel in vitro model would greatly enhance our understanding of cancer progression and treatment resistance. In this work, we present such a model, based simply on applying standard pluripotency/embryonic stem cell media alone. Core pluripotency stem cell master regulators (OCT4, SOX2 and NANOG) along with epithelial-mesenchymal transition (EMT) markers (Snail, Slug, vimentin and N-cadherin) were induced in human prostate, breast, lung, bladder, colorectal, and renal cancer cells. RNA sequencing revealed pathways activated by pluripotency inducing culture that were shared across all cancers examined. These pathways highlight a potential core mechanism of treatment resistance. With a focus on prostate cancer, the culture-based induction of core pluripotent stem cell regulators was shown to promote survival in castrate conditions-mimicking first line treatment resistance with hormonal therapies. This acquired phenotype was shown to be mediated through the upregulation of iodothyronine deiodinase DIO2, a critical modulator of the thyroid hormone signalling pathway. Subsequent inhibition of DIO2 was shown to supress expression of prostate specific antigen, the cardinal clinical biomarker of prostate cancer progression and highlighted a novel target for clinical translation in this otherwise fatal disease. This study identifies a new and widely accessible simple preclinical model to recreate and explore underpinning pathways of lethal disease and treatment resistance.
中文翻译:

癌症中核心多能性主调节因子的诱导定义了不良的临床结果和治疗耐药性。
干细胞特征与许多癌症类型的治疗耐药性和不良预后有关。在新型体外模型中诱导和调节维持致命癌症的这些特征标志的途径的能力将大大增强我们对癌症进展和治疗耐药性的理解。在这项工作中,我们提出了这样一个模型,它仅基于单独应用标准多能性/胚胎干细胞培养基。核心多能干细胞主调节因子(OCT4、SOX2 和 NANOG)以及上皮-间质转化 (EMT) 标记物(Snail、Slug、波形蛋白和 N-钙粘蛋白)在人前列腺、乳腺、肺、膀胱、结肠直肠和肾脏中被诱导癌细胞。RNA 测序揭示了由多能性诱导培养激活的通路,这些通路在所有检查的癌症中都是共享的。这些途径突出了治疗耐药性的潜在核心机制。以前列腺癌为重点,基于培养的核心多能干细胞调节剂的诱导被证明可以促进阉割条件下的存活——模拟激素疗法的一线治疗耐药性。这种获得性表型被证明是通过碘甲腺原氨酸脱碘酶 DIO2 的上调介导的,DIO2 是甲状腺激素信号通路的关键调节剂。随后抑制 DIO2 被证明可以抑制前列腺特异性抗原的表达,前列腺特异性抗原是前列腺癌进展的主要临床生物标志物,并突出了这种致命疾病临床转化的新靶点。
更新日期:2019-02-13
中文翻译:

癌症中核心多能性主调节因子的诱导定义了不良的临床结果和治疗耐药性。
干细胞特征与许多癌症类型的治疗耐药性和不良预后有关。在新型体外模型中诱导和调节维持致命癌症的这些特征标志的途径的能力将大大增强我们对癌症进展和治疗耐药性的理解。在这项工作中,我们提出了这样一个模型,它仅基于单独应用标准多能性/胚胎干细胞培养基。核心多能干细胞主调节因子(OCT4、SOX2 和 NANOG)以及上皮-间质转化 (EMT) 标记物(Snail、Slug、波形蛋白和 N-钙粘蛋白)在人前列腺、乳腺、肺、膀胱、结肠直肠和肾脏中被诱导癌细胞。RNA 测序揭示了由多能性诱导培养激活的通路,这些通路在所有检查的癌症中都是共享的。这些途径突出了治疗耐药性的潜在核心机制。以前列腺癌为重点,基于培养的核心多能干细胞调节剂的诱导被证明可以促进阉割条件下的存活——模拟激素疗法的一线治疗耐药性。这种获得性表型被证明是通过碘甲腺原氨酸脱碘酶 DIO2 的上调介导的,DIO2 是甲状腺激素信号通路的关键调节剂。随后抑制 DIO2 被证明可以抑制前列腺特异性抗原的表达,前列腺特异性抗原是前列腺癌进展的主要临床生物标志物,并突出了这种致命疾病临床转化的新靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号