Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53.
Oncogene ( IF 6.9 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41388-019-0743-4 Li Zhang 1 , Weilin Li 1, 2 , Lei Cao 1 , Jiaying Xu 1 , Yun Qian 3 , Huarong Chen 1 , Yanquan Zhang 1 , Wei Kang 4 , Hongyan Gou 1 , Chi Chun Wong 1 , Jun Yu 1
Oncogene ( IF 6.9 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41388-019-0743-4 Li Zhang 1 , Weilin Li 1, 2 , Lei Cao 1 , Jiaying Xu 1 , Yun Qian 3 , Huarong Chen 1 , Yanquan Zhang 1 , Wei Kang 4 , Hongyan Gou 1 , Chi Chun Wong 1 , Jun Yu 1
Affiliation
|
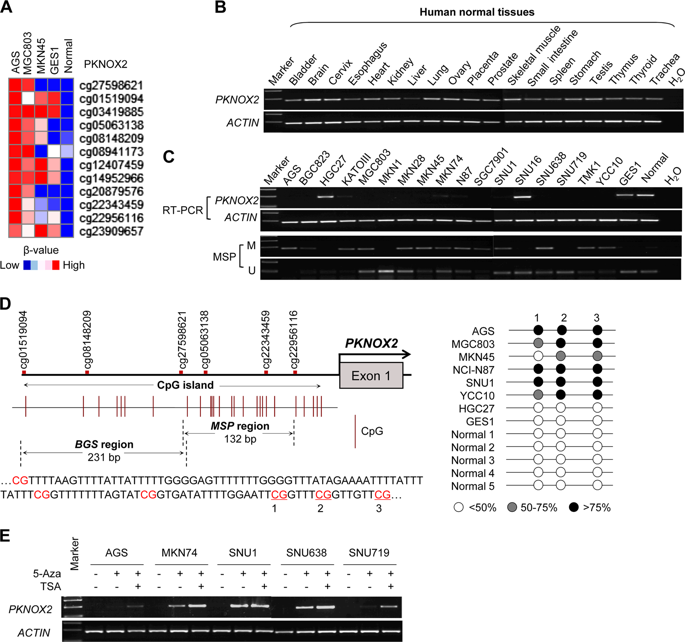
Promoter methylation plays a vital role in tumorigenesis through transcriptional silencing of tumor suppressive genes. Using genome-wide methylation array, we first identified PBX/Knotted Homeobox 2 (PKNOX2) as a candidate tumor suppressor in gastric cancer. PKNOX2 mRNA expression is largely silenced in gastric cancer cell lines and primary gastric cancer via promoter methylation. Promoter methylation of PKNOX2 was associated with poor survival in gastric cancer patients. A series of in vitro and in vivo functional studies revealed that PKNOX2 functions as a tumor suppressor. Ectopic PKNOX2 expression inhibited cell proliferation in GC cell lines and suppressed growth of tumor xenografts in mice via induction of apoptosis and cell cycle arrest; and suppressed cell migration and invasion by blocking epithelial-to-mesenchymal transition. On the other hand, knockdown PKNOX2 in normal gastric epithelial cells triggered diverse malignant phenotypes. Mechanistically, PKNOX2 exerts its tumor suppressive effect by promoting the up-regulation of Insulin like Growth Factor Binding Protein 5 (IGFBP5) and TP53. PKNOX2 binds to the promoter regions of IGFBP5 and TP53 and transcriptionally activated their expression by chromatin immunoprecipitation (ChIP)-PCR assay. IGFBP5 knockdown partly abrogated tumor suppressive effect of PKNOX2, indicating that the function(s) of PKNOX2 are dependent on IGFBP5. IGFBP5 promoted PKNOX2-mediated up-regulation of p53. As a consequence, p53 transcription target genes were coordinately up-regulated in PKNOX2-expressing GC cells, leading to tumor suppression. In summary, our results identified PKNOX2 as a tumor suppressor in gastric cancer by activation of IGFBP5 and p53 signaling pathways. PKNOX2 promoter hypermethylation might be a biomarker for the poor survival of gastric cancer patients.
中文翻译:

PKNOX2通过IGFBP5和p53的转录激活抑制胃癌。
通过抑制肿瘤基因的转录沉默,启动子甲基化在肿瘤发生中起着至关重要的作用。使用全基因组甲基化阵列,我们首先确定了PBX / Knotted Homeobox 2(PKNOX2)作为胃癌的候选肿瘤抑制因子。PKNOX2 mRNA表达在胃癌细胞系和原发性胃癌中通过启动子甲基化被大大沉默。PKNOX2的启动子甲基化与胃癌患者生存不良有关。一系列的体外和体内功能研究表明,PKNOX2发挥抑癌作用。异位PKNOX2的表达通过诱导凋亡和细胞周期阻滞,抑制了GC细胞系中的细胞增殖,并抑制了小鼠肿瘤异种移植物的生长;并通过阻止上皮到间充质细胞的转化来抑制细胞的迁移和侵袭。另一方面,正常胃上皮细胞中的敲低PKNOX2触发了多种恶性表型。从机制上讲,PKNOX2通过促进胰岛素样生长因子结合蛋白5(IGFBP5)和TP53的上调发挥其肿瘤抑制作用。PKNOX2与IGFBP5和TP53的启动子区域结合,并通过染色质免疫沉淀(ChIP)-PCR分析转录激活它们的表达。IGFBP5敲低部分废除了PKNOX2的肿瘤抑制作用,表明PKNOX2的功能依赖于IGFBP5。IGFBP5促进PKNOX2介导的p53上调。结果,在表达PKNOX2的GC细胞中p53转录靶基因被协同上调,从而导致肿瘤抑制。总之,我们的研究结果通过激活IGFBP5和p53信号通路,将PKNOX2鉴定为胃癌的抑癌基因。PKNOX2启动子高甲基化可能是胃癌患者生存不良的生物标志物。
更新日期:2019-02-13
中文翻译:

PKNOX2通过IGFBP5和p53的转录激活抑制胃癌。
通过抑制肿瘤基因的转录沉默,启动子甲基化在肿瘤发生中起着至关重要的作用。使用全基因组甲基化阵列,我们首先确定了PBX / Knotted Homeobox 2(PKNOX2)作为胃癌的候选肿瘤抑制因子。PKNOX2 mRNA表达在胃癌细胞系和原发性胃癌中通过启动子甲基化被大大沉默。PKNOX2的启动子甲基化与胃癌患者生存不良有关。一系列的体外和体内功能研究表明,PKNOX2发挥抑癌作用。异位PKNOX2的表达通过诱导凋亡和细胞周期阻滞,抑制了GC细胞系中的细胞增殖,并抑制了小鼠肿瘤异种移植物的生长;并通过阻止上皮到间充质细胞的转化来抑制细胞的迁移和侵袭。另一方面,正常胃上皮细胞中的敲低PKNOX2触发了多种恶性表型。从机制上讲,PKNOX2通过促进胰岛素样生长因子结合蛋白5(IGFBP5)和TP53的上调发挥其肿瘤抑制作用。PKNOX2与IGFBP5和TP53的启动子区域结合,并通过染色质免疫沉淀(ChIP)-PCR分析转录激活它们的表达。IGFBP5敲低部分废除了PKNOX2的肿瘤抑制作用,表明PKNOX2的功能依赖于IGFBP5。IGFBP5促进PKNOX2介导的p53上调。结果,在表达PKNOX2的GC细胞中p53转录靶基因被协同上调,从而导致肿瘤抑制。总之,我们的研究结果通过激活IGFBP5和p53信号通路,将PKNOX2鉴定为胃癌的抑癌基因。PKNOX2启动子高甲基化可能是胃癌患者生存不良的生物标志物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号