Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Kinase N1 control of androgen-responsive serum response factor action provides rationale for novel prostate cancer treatment strategy.
Oncogene ( IF 6.9 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41388-019-0732-7 Varadha Balaji Venkadakrishnan 1, 2 , Adam D DePriest 3 , Sangeeta Kumari 1 , Dhirodatta Senapati 1 , Salma Ben-Salem 1 , Yixue Su 1 , Giridhar Mudduluru 1 , Qiang Hu 4 , Eduardo Cortes 4 , Elena Pop 5 , James L Mohler 5 , Gissou Azabdaftari 5, 6 , Kristopher Attwood 4 , Rajal B Shah 7 , Christina Jamieson 8 , Scott M Dehm 9 , Cristina Magi-Galluzzi 7 , Eric Klein 10 , Nima Sharifi 1, 10, 11 , Song Liu 4 , Hannelore V Heemers 1, 10, 11
Oncogene ( IF 6.9 ) Pub Date : 2019-02-11 , DOI: 10.1038/s41388-019-0732-7 Varadha Balaji Venkadakrishnan 1, 2 , Adam D DePriest 3 , Sangeeta Kumari 1 , Dhirodatta Senapati 1 , Salma Ben-Salem 1 , Yixue Su 1 , Giridhar Mudduluru 1 , Qiang Hu 4 , Eduardo Cortes 4 , Elena Pop 5 , James L Mohler 5 , Gissou Azabdaftari 5, 6 , Kristopher Attwood 4 , Rajal B Shah 7 , Christina Jamieson 8 , Scott M Dehm 9 , Cristina Magi-Galluzzi 7 , Eric Klein 10 , Nima Sharifi 1, 10, 11 , Song Liu 4 , Hannelore V Heemers 1, 10, 11
Affiliation
|
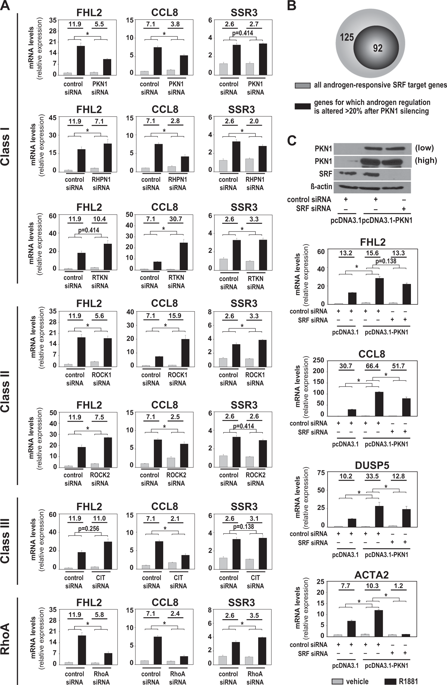
Sustained reliance on androgen receptor (AR) after failure of AR-targeting androgen deprivation therapy (ADT) prevents effective treatment of castration-recurrent (CR) prostate cancer (CaP). Interfering with the molecular machinery by which AR drives CaP progression may be an alternative therapeutic strategy but its feasibility remains to be tested. Here, we explore targeting the mechanism by which AR, via RhoA, conveys androgen-responsiveness to serum response factor (SRF), which controls aggressive CaP behavior and is maintained in CR-CaP. Following a siRNA screen and candidate gene approach, RNA-Seq studies confirmed that the RhoA effector Protein Kinase N1 (PKN1) transduces androgen-responsiveness to SRF. Androgen treatment induced SRF-PKN1 interaction, and PKN1 knockdown or overexpression severely impaired or stimulated, respectively, androgen regulation of SRF target genes. PKN1 overexpression occurred during clinical CR-CaP progression, and hastened CaP growth and shortened CR-CaP survival in orthotopic CaP xenografts. PKN1's effects on SRF relied on its kinase domain. The multikinase inhibitor lestaurtinib inhibited PKN1 action and preferentially affected androgen regulation of SRF over direct AR target genes. In a CR-CaP patient-derived xenograft, expression of SRF target genes was maintained while AR target gene expression declined and proliferative gene expression increased. PKN1 inhibition decreased viability of CaP cells before and after ADT. In patient-derived CaP explants, lestaurtinib increased AR target gene expression but did not significantly alter SRF target gene or proliferative gene expression. These results provide proof-of-principle for selective forms of ADT that preferentially target different fractions of AR's transcriptional output to inhibit CaP growth.
中文翻译:

蛋白激酶N1对雄激素反应性血清反应因子作用的控制为新型前列腺癌治疗策略提供了理论依据。
靶向AR的雄激素剥夺治疗(ADT)失败后,持续依赖雄激素受体(AR)会阻止去势复发(CR)前列腺癌(CaP)的有效治疗。干扰AR驱动CaP进程的分子机制可能是另一种治疗策略,但其可行性仍有待测试。在这里,我们探索靶向机制,AR通过RhoA传达雄激素对血清反应因子(SRF)的反应,该反应因子控制侵略性的CaP行为并维持在CR-CaP中。继siRNA筛选和候选基因方法后,RNA-Seq研究证实RhoA效应蛋白激酶N1(PKN1)转导了雄激素对SRF的反应性。雄激素治疗分别导致SRF-PKN1相互作用和PKN1敲低或过表达,严重受损或刺激,SRF靶基因的雄激素调节。在原位CaP异种移植中,PKN1过表达发生在临床CR-CaP进程中,并加速了CaP的生长并缩短了CR-CaP的存活时间。PKN1对SRF的作用依赖于其激酶结构域。与直接AR靶基因相比,多激酶抑制剂lestaurtinib抑制PKN1作用并优先影响SRF的雄激素调节。在来自CR-CaP患者的异种移植物中,SRF靶基因的表达得以维持,而AR靶基因的表达下降而增生性基因的表达增加。PKN1抑制会降低ADT之前和之后CaP细胞的活力。在患者来源的CaP外植体中,来舒替尼增加了AR靶基因的表达,但并未显着改变SRF靶基因或增生性基因的表达。
更新日期:2019-02-11
中文翻译:

蛋白激酶N1对雄激素反应性血清反应因子作用的控制为新型前列腺癌治疗策略提供了理论依据。
靶向AR的雄激素剥夺治疗(ADT)失败后,持续依赖雄激素受体(AR)会阻止去势复发(CR)前列腺癌(CaP)的有效治疗。干扰AR驱动CaP进程的分子机制可能是另一种治疗策略,但其可行性仍有待测试。在这里,我们探索靶向机制,AR通过RhoA传达雄激素对血清反应因子(SRF)的反应,该反应因子控制侵略性的CaP行为并维持在CR-CaP中。继siRNA筛选和候选基因方法后,RNA-Seq研究证实RhoA效应蛋白激酶N1(PKN1)转导了雄激素对SRF的反应性。雄激素治疗分别导致SRF-PKN1相互作用和PKN1敲低或过表达,严重受损或刺激,SRF靶基因的雄激素调节。在原位CaP异种移植中,PKN1过表达发生在临床CR-CaP进程中,并加速了CaP的生长并缩短了CR-CaP的存活时间。PKN1对SRF的作用依赖于其激酶结构域。与直接AR靶基因相比,多激酶抑制剂lestaurtinib抑制PKN1作用并优先影响SRF的雄激素调节。在来自CR-CaP患者的异种移植物中,SRF靶基因的表达得以维持,而AR靶基因的表达下降而增生性基因的表达增加。PKN1抑制会降低ADT之前和之后CaP细胞的活力。在患者来源的CaP外植体中,来舒替尼增加了AR靶基因的表达,但并未显着改变SRF靶基因或增生性基因的表达。











































 京公网安备 11010802027423号
京公网安备 11010802027423号