当前位置:
X-MOL 学术
›
npj Prim. Care Respir. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of adverse events associated with different spacers used with non-extrafine beclometasone dipropionate for asthma.
npj Primary Care Respiratory Medicine ( IF 3.1 ) Pub Date : 2019-02-08 , DOI: 10.1038/s41533-019-0115-0 Simon Wan Yau Ming 1 , John Haughney 2 , Dermot Ryan 3, 4 , Shishir Patel 5 , Matthias Ochel 5 , Martina Stagno d'Alcontres 1 , Susannah Thornhill 1 , Janwillem W H Kocks 1, 6 , David Price 1, 2, 3
npj Primary Care Respiratory Medicine ( IF 3.1 ) Pub Date : 2019-02-08 , DOI: 10.1038/s41533-019-0115-0 Simon Wan Yau Ming 1 , John Haughney 2 , Dermot Ryan 3, 4 , Shishir Patel 5 , Matthias Ochel 5 , Martina Stagno d'Alcontres 1 , Susannah Thornhill 1 , Janwillem W H Kocks 1, 6 , David Price 1, 2, 3
Affiliation
|
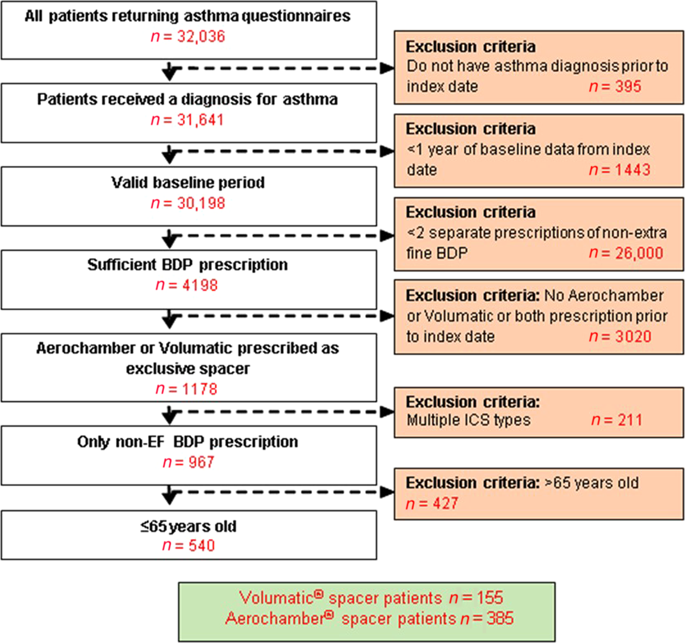
Co-prescription of Aerochamber® spacer with non-extrafine beclometasone diproprionate (non-EF BDP) is common but unlicensed. We report a comparison of inhaled corticosteroid (ICS)-related adverse events between patients co-prescribed Aerochamber compared to the licensed Volumatic® spacer. We utilised two historical cohorts: questionnaire-based and electronic medical record (EMR)-based, to assess patient-reported and EMR-recorded adverse events in patients with asthma prescribed non-EF BDP. Marginal effect estimate (MEE) was calculated to determine non-inferiority of Aerochamber compared to Volumatic in terms of patient-reported oral thrush and hoarseness with margin of 0.13. Other patient-reported adverse events (sore throat, bruising, weight gain, and coughing), and EMR-recorded adverse events were also assessed. Rate of patient-reported oral adverse events were non-inferior in 385 patients prescribed Aerochamber compared to 155 patients prescribed Volumatic (27.7 vs 29.9%; MEE, -0.043; 95% CI, -0.133 to 0.047). Total patient-reported adverse events did not differ significantly between Aerochamber and Volumatic (53.3 vs 49.7% with ≥1 adverse event). The EMR-based study of 1471 matched pairs of subjects did not show significantly different number of EMR-recorded adverse events between Aerochamber and Volumatic (12.5 vs 12.8% with ≥1 adverse events). Co-prescribing Aerochamber with non-EF BDP does not increase the risk for patient-reported and EMR-recorded ICS-related adverse events compared to co-prescribing Volumatic.
中文翻译:

比较与非特细贝氯米松二丙酸酯用于哮喘的不同间隔物相关的不良事件。
Aerochamber®垫片与非特细双氯贝米松双丙酸酯(非EF BDP)的共同处方很常见,但未经许可。我们报告了与处方许可的Volumatic®垫片相比,患者共同处方的Aerochamber吸入皮质类固醇(ICS)相关的不良事件的比较。我们利用两个历史队列:基于问卷调查和基于电子病历(EMR)来评估哮喘处方非EF BDP患者的患者报告和EMR记录的不良事件。根据患者报告的口腔鹅口疮和声音嘶哑,以0.13的余量计算了边际效应估计值(MEE),以确定Aerochamber与Volumatic相比是否具有劣质性。还评估了其他患者报告的不良事件(咽痛,瘀伤,体重增加和咳嗽),以及EMR记录的不良事件。在385例使用Aerochamber的患者中,患者报告的口腔不良事件发生率不逊于155例使用Volumatic的患者(27.7 vs 29.9%; MEE,-0.043; 95%CI,-0.133至0.047)。Aerochamber和Volumatic之间由患者报告的总不良事件没有显着差异(不良事件≥1的患者分别为53.3 vs 49.7%)。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。043; 95%CI,-0.133至0.047)。Aerochamber和Volumatic之间由患者报告的总不良事件没有显着差异(不良事件≥1的患者分别为53.3 vs 49.7%)。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。043; 95%CI,-0.133至0.047)。Aerochamber和Volumatic之间由患者报告的总不良事件没有显着差异(不良事件≥1的患者分别为53.3 vs 49.7%)。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。
更新日期:2019-11-18
中文翻译:

比较与非特细贝氯米松二丙酸酯用于哮喘的不同间隔物相关的不良事件。
Aerochamber®垫片与非特细双氯贝米松双丙酸酯(非EF BDP)的共同处方很常见,但未经许可。我们报告了与处方许可的Volumatic®垫片相比,患者共同处方的Aerochamber吸入皮质类固醇(ICS)相关的不良事件的比较。我们利用两个历史队列:基于问卷调查和基于电子病历(EMR)来评估哮喘处方非EF BDP患者的患者报告和EMR记录的不良事件。根据患者报告的口腔鹅口疮和声音嘶哑,以0.13的余量计算了边际效应估计值(MEE),以确定Aerochamber与Volumatic相比是否具有劣质性。还评估了其他患者报告的不良事件(咽痛,瘀伤,体重增加和咳嗽),以及EMR记录的不良事件。在385例使用Aerochamber的患者中,患者报告的口腔不良事件发生率不逊于155例使用Volumatic的患者(27.7 vs 29.9%; MEE,-0.043; 95%CI,-0.133至0.047)。Aerochamber和Volumatic之间由患者报告的总不良事件没有显着差异(不良事件≥1的患者分别为53.3 vs 49.7%)。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。043; 95%CI,-0.133至0.047)。Aerochamber和Volumatic之间由患者报告的总不良事件没有显着差异(不良事件≥1的患者分别为53.3 vs 49.7%)。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。043; 95%CI,-0.133至0.047)。Aerochamber和Volumatic之间由患者报告的总不良事件没有显着差异(不良事件≥1的患者分别为53.3 vs 49.7%)。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。基于EMR的1471对配对受试者的研究并未显示Aerochamber和Volumatic之间EMR记录的不良事件数量有显着差异(不良事件≥1时为12.5对12.8%)。与共同处方Volumatic相比,与非EF BDP共同处方Aerochamber不会增加患者报告和EMR记录的ICS相关不良事件的风险。










































 京公网安备 11010802027423号
京公网安备 11010802027423号