npj Vaccines ( IF 9.2 ) Pub Date : 2019-02-08 , DOI: 10.1038/s41541-019-0102-z Genevieve M. Weir , Lisa D. MacDonald , Rajkannan Rajagopalan , Gloria S. Sivko , Michelle W. Valderas , Jonathan Rayner , Bradley J. Berger , Leeladhar Sammatur , Marianne M. Stanford
|
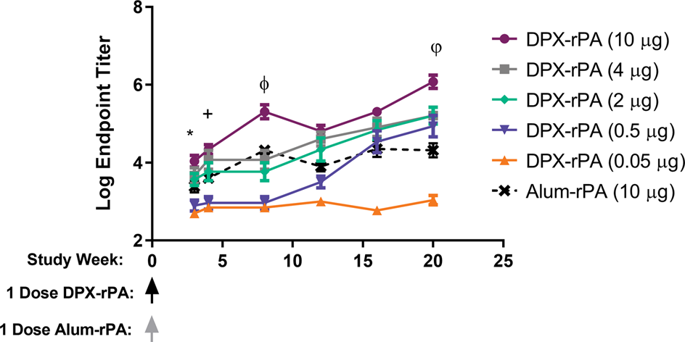
Anthrax is a serious biological threat caused by pulmonary exposure to aerosolized spores of Bacillus anthracis. Biothrax® (anthrax vaccine adsorbed (AVA)) is the only Food and Drug Administration-licensed vaccine and requires five administrations over 12 months with annual boosting to maintain pre-exposure prophylaxis. Here we report the evaluation of a single intramuscular injection of recombinant B. anthracis-protective antigen (rPA) formulated in the DPX delivery platform. Immune responses were compared to an alum-based formulation in mice and rabbits. Serological analysis of anti-rPA immunoglobulin G and toxin neutralization activity demonstrated higher responses induced by DPX-rPA when compared to rPA in alum. DPX-rPA was compared to AVA in rabbits and non-human primates (NHPs). In both species, DPX-rPA generated responses after a single immunization, whereas AVA required two immunizations. In rabbits, single injection of DPX-rPA or two injections of AVA conferred 100% protection from anthrax challenge. In NHPs, single-dose DPX-rPA was 100% protective against challenge, whereas one animal in the two-dose AVA group and all saline administered animals succumbed to infection. DPX-rPA was minimally reactogenic in all species tested. These data indicate that DPX-rPA may offer improvement over AVA by reducing the doses needed for protective immune responses and is a promising candidate as a new-generation anthrax vaccine.
中文翻译:

单剂量的DPX-rPA(一种增强递送的炭疽疫苗制剂)可防止致命的炭疽芽孢杆菌孢子吸入挑战
炭疽是由肺暴露于炭疽芽孢杆菌的气雾化孢子引起的严重生物威胁。的BioThrax ®(炭疽吸附疫苗(AVA))是唯一的食品和药品监督管理局批准的疫苗,需要超过12个月5个主管部门每年提高保持暴露前预防。在这里,我们报告对重组炭疽芽孢杆菌的单次肌内注射的评估-在DPX输送平台中配制的保护性抗原(rPA)。在小鼠和兔子中,将免疫应答与基于明矾的制剂进行了比较。血清中抗rPA免疫球蛋白G和毒素中和活性的血清学分析表明,与明矾中的rPA相比,DPX-rPA诱导的应答更高。在兔和非人灵长类动物(NHP)中,将DPX-rPA与AVA进行了比较。在两个物种中,DPX-rPA在单次免疫后均会产生反应,而AVA需要两次免疫。在兔子中,单次注射DPX-rPA或两次注射AVA可以100%保护免受炭疽的侵害。在NHP中,单剂量DPX-rPA对攻击具有100%的保护作用,而两剂量AVA组中的一只动物和所有用生理盐水给药的动物都容易感染。在所有测试的物种中,DPX-rPA的反应原性最低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号