当前位置:
X-MOL 学术
›
npj Biofilms Microbiomes
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Citrullination mediated by PPAD constrains biofilm formation in P. gingivalis strain 381.
npj Biofilms and Microbiomes ( IF 7.8 ) Pub Date : 2019-02-07 , DOI: 10.1038/s41522-019-0081-x Danielle M Vermilyea 1 , Gregory K Ottenberg 1 , Mary E Davey 1
npj Biofilms and Microbiomes ( IF 7.8 ) Pub Date : 2019-02-07 , DOI: 10.1038/s41522-019-0081-x Danielle M Vermilyea 1 , Gregory K Ottenberg 1 , Mary E Davey 1
Affiliation
|
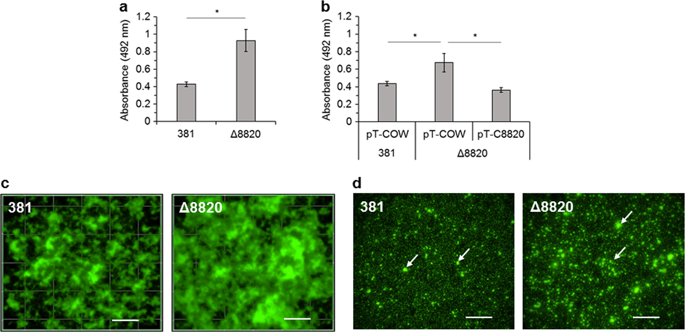
Porphyromonas gingivalis is the only known human-associated prokaryote that produces a peptidylarginine deiminase (PPAD), a protein-modifying enzyme that is secreted along with a number of virulence factors via a type IX secretion system (T9SS). While the function of PPAD in P. gingivalis physiology is not clear, human peptidylarginine deiminases are known to convert positively charged arginine residues within proteins to neutral citrulline and, thereby, impact protein conformation and function. Here, we report that the lack of citrullination in a PPAD deletion mutant (Δ8820) enhances biofilm formation. More Δ8820 cells attached to the surface than the parent strain during the early stages of biofilm development and, ultimately, mature Δ8820 biofilms were comprised of significantly more cell-cell aggregates and extracellular matrix. Imaging by electron microscopy discovered that Δ8820 biofilm cells secrete copious amounts of protein aggregates. Furthermore, gingipain-derived adhesin proteins, which are also secreted by the T9SS were predicted by mass spectrometry to be citrullinated and citrullination of these targets by wild-type strain 381 in vitro was confirmed. Lastly, Δ8820 biofilms contained more gingipain-derived adhesin proteins and more gingipain activity than 381 biofilms. Overall, our findings support the model that citrullination of T9SS cargo proteins known to play a key role in colonization, such as gingipain-derived adhesin proteins, is an underlying mechanism that modulates P. gingivalis biofilm development.
中文翻译:

PPAD 介导的瓜氨酸化限制牙龈卟啉单胞菌菌株 381 中生物膜的形成。
牙龈卟啉单胞菌是唯一已知的产生肽基精氨酸脱亚胺酶 (PPAD) 的人类相关原核生物,PPAD 是一种蛋白质修饰酶,通过 IX 型分泌系统 (T9SS) 与许多毒力因子一起分泌。虽然 PPAD 在牙龈卟啉单胞菌生理学中的功能尚不清楚,但已知人肽基精氨酸脱亚氨酶可将蛋白质内带正电荷的精氨酸残基转化为中性瓜氨酸,从而影响蛋白质构象和功能。在这里,我们报告说,PPAD 缺失突变体 (Δ8820) 缺乏瓜氨酸化会增强生物膜的形成。在生物膜形成的早期阶段,比亲本菌株更多的Δ8820细胞附着在表面,最终,成熟的Δ8820生物膜由明显更多的细胞-细胞聚集体和细胞外基质组成。电子显微镜成像发现 Δ8820 生物膜细胞分泌大量蛋白质聚集体。此外,通过质谱分析预测也由 T9SS 分泌的牙龈蛋白酶衍生的粘附素蛋白将被瓜氨酸化,并证实野生型菌株 381 在体外对这些靶标进行瓜氨酸化。最后,Δ8820 生物膜比 381 生物膜含有更多的牙龈蛋白酶衍生的粘附素蛋白和更多的牙龈蛋白酶活性。总体而言,我们的研究结果支持这样的模型:已知在定植中起关键作用的 T9SS 货物蛋白(例如牙龈蛋白酶衍生的粘附蛋白)的瓜氨酸化是调节牙龈卟啉单胞菌生物膜发育的潜在机制。
更新日期:2019-02-07
中文翻译:

PPAD 介导的瓜氨酸化限制牙龈卟啉单胞菌菌株 381 中生物膜的形成。
牙龈卟啉单胞菌是唯一已知的产生肽基精氨酸脱亚胺酶 (PPAD) 的人类相关原核生物,PPAD 是一种蛋白质修饰酶,通过 IX 型分泌系统 (T9SS) 与许多毒力因子一起分泌。虽然 PPAD 在牙龈卟啉单胞菌生理学中的功能尚不清楚,但已知人肽基精氨酸脱亚氨酶可将蛋白质内带正电荷的精氨酸残基转化为中性瓜氨酸,从而影响蛋白质构象和功能。在这里,我们报告说,PPAD 缺失突变体 (Δ8820) 缺乏瓜氨酸化会增强生物膜的形成。在生物膜形成的早期阶段,比亲本菌株更多的Δ8820细胞附着在表面,最终,成熟的Δ8820生物膜由明显更多的细胞-细胞聚集体和细胞外基质组成。电子显微镜成像发现 Δ8820 生物膜细胞分泌大量蛋白质聚集体。此外,通过质谱分析预测也由 T9SS 分泌的牙龈蛋白酶衍生的粘附素蛋白将被瓜氨酸化,并证实野生型菌株 381 在体外对这些靶标进行瓜氨酸化。最后,Δ8820 生物膜比 381 生物膜含有更多的牙龈蛋白酶衍生的粘附素蛋白和更多的牙龈蛋白酶活性。总体而言,我们的研究结果支持这样的模型:已知在定植中起关键作用的 T9SS 货物蛋白(例如牙龈蛋白酶衍生的粘附蛋白)的瓜氨酸化是调节牙龈卟啉单胞菌生物膜发育的潜在机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号