Oncogene ( IF 8 ) Pub Date : 2019-02-01 , DOI: 10.1038/s41388-019-0706-9 Martin Fischer
|
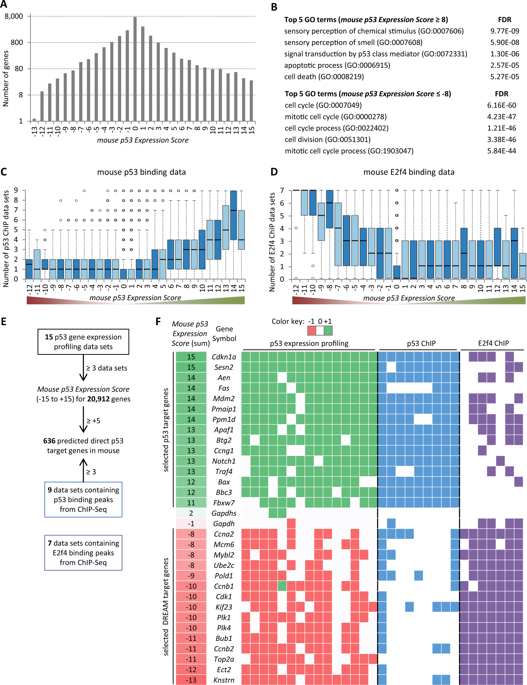
Understanding the p53 tumor suppressor pathway remains crucial for the design of anticancer strategies. Studies in human tumors and mouse models help to unravel the molecular mechanisms that underlie the p53 signaling pathway. Yet, the p53 gene regulatory network (GRN) is not the same in mice and humans. The comparison of the regulatory networks of p53 in mice and humans reveals that gene up- and down-regulation by p53 are distinctly affected during evolution. Importantly, gene up-regulation by p53 underwent more rapid evolution and gene down-regulation has been evolutionarily constrained. This difference stems from the two major mechanisms employed by p53 to regulate gene expression: up-regulation through direct p53 target gene binding and indirect down-regulation through the p53-p21-DREAM pathway. More than 1000 genes have been identified to differ in their p53-dependent expression between mice and humans. Analysis of p53 gene expression profiles and p53 binding data reveal that turnover of p53 binding sites is the major mechanism underlying extensive variation in p53-dependent gene up-regulation. Only a core set of high-confidence genes appears to be directly regulated by p53 in both species. In contrast to up-regulation, p53-induced down-regulation is well conserved between mice and humans and controls cell cycle genes. Here a curated data set is provided that extends the previously established web-atlas at www.targetgenereg.org to assess the p53 response of any human gene of interest and its mouse ortholog. Taken together, the analysis reveals a limited translation potential from mouse models to humans for the p53 GRN.
中文翻译:

小鼠与人之间p53基因调控网络的保守性和差异性
了解p53抑癌途径对于设计抗癌策略仍然至关重要。对人类肿瘤和小鼠模型的研究有助于揭示构成p53信号通路基础的分子机制。但是,p53基因调控网络(GRN)在小鼠和人类中并不相同。小鼠和人类中p53调控网络的比较表明,p53基因的上调和下调在进化过程中受到明显影响。重要的是,通过p53进行的基因上调经历了更快的进化,并且基因的下调在进化上受到了限制。这种差异源于p53用来调节基因表达的两种主要机制:通过直接p53靶基因结合的上调和通过p53-p21-DREAM途径的间接下调。已经鉴定出超过1000个基因在小鼠和人类之间的p53依赖性表达方面存在差异。对p53基因表达谱和p53结合数据的分析表明,p53结合位点的转换是p53依赖性基因上调广泛变化的主要机制。在这两个物种中,只有一组核心的高信度基因似乎直接受p53调控。与上调相反,p53诱导的下调在小鼠和人类之间非常保守,并控制细胞周期基因。这里提供了一个精选的数据集,该数据集扩展了先前在www.targetgenereg.org上建立的网络地图集,以评估任何人类感兴趣的基因及其小鼠直系同源基因的p53反应。两者合计,该分析揭示了从小鼠模型到人的p53 GRN的翻译潜力有限。



























 京公网安备 11010802027423号
京公网安备 11010802027423号