当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brown Carbon Formation from Nighttime Chemistry of Unsaturated Heterocyclic Volatile Organic Compounds
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2019-02-05 , DOI: 10.1021/acs.estlett.9b00017 Huanhuan Jiang 1 , Alexander L. Frie 1 , Avi Lavi 2 , Jin Y. Chen 3 , Haofei Zhang 2, 3 , Roya Bahreini 1, 2, 3 , Ying-Hsuan Lin 1, 3
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2019-02-05 , DOI: 10.1021/acs.estlett.9b00017 Huanhuan Jiang 1 , Alexander L. Frie 1 , Avi Lavi 2 , Jin Y. Chen 3 , Haofei Zhang 2, 3 , Roya Bahreini 1, 2, 3 , Ying-Hsuan Lin 1, 3
Affiliation
|
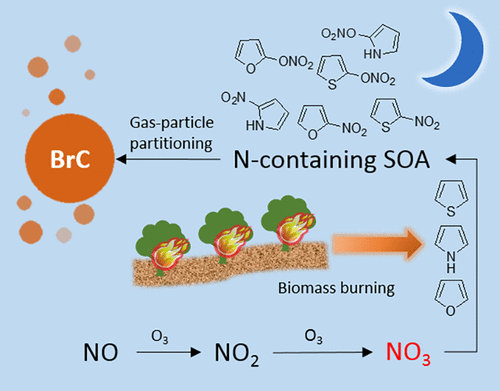
Nighttime atmospheric processing enhances the formation of brown carbon aerosol (BrC) in biomass burning plumes. Heterocyclic compounds, a group of volatile organic compounds (VOCs) abundant in biomass burning smoke, are possible BrC sources. Here, we investigated the nitrate radical (NO3)-initiated oxidation of three unsaturated heterocyclic compounds (pyrrole, furan, and thiophene) as a source of BrC. The imaginary component of the refractive index at 375 nm (k375), the single scattering albedo at 375 nm (SSA375), and average mass absorption coefficients (⟨MAC⟩290–700 nm) of the resulting secondary organic aerosol (SOA) are reported. Compared to furan and thiophene, NO3 oxidation of pyrrole has the highest SOA yield. Pyrrole SOA (k375 = 0.015 ± 0.003, SSA = 0.86 ± 0.01, ⟨MAC⟩290–700 nm = 3400 ± 700 cm2 g–1) is also more absorbing than furan SOA (⟨MAC⟩290–700 nm = 1100 ± 200 cm2 g–1) and thiophene SOA (k375 = 0.003 ± 0.002, SSA375 = 0.98 ± 0.01, ⟨MAC⟩290–700 nm = 3000 ± 500 cm2 g–1). Compared to other SOA systems, MACs reported in this study are higher than those from biogenic precursors and similar to high-NOx anthropogenic SOA. Characterization of SOA molecular composition using high-resolution mass spectrometric measurements revealed unsaturated heterocyclic nitro products or organonitrates as possible chromophores in BrC from all three precursors. These findings reveal nighttime oxidation of fire-sourced heterocyclic compounds, particularly pyrrole, as a plausible source of BrC.
中文翻译:

不饱和杂环挥发性有机化合物夜间化学过程中形成的褐碳
夜间大气处理会增强生物质燃烧烟羽中棕色碳气溶胶(BrC)的形成。杂环化合物可能是BrC的来源,杂环化合物是在燃烧生物质的烟雾中丰富的一组挥发性有机化合物(VOC)。在这里,我们研究了作为BrC来源的三种不饱和杂环化合物(吡咯,呋喃和噻吩)的硝酸根(NO 3)引发的氧化。所产生的二次有机气溶胶(SOA)在375 nm(k 375)处的折射率的虚部,在375 nm(SSA 375)处的单散射反照率和平均质量吸收系数(⟨MAC⟩290–700 nm)被报道。与呋喃和噻吩相比,NO 3吡咯的氧化具有最高的SOA产量。吡咯SOA(k 375 = 0.015±0.003,SSA = 0.86±0.01,⟨MAC⟩290–700 nm = 3400±700 cm 2 g –1)也比呋喃SOA(⟨MAC⟩290–700 nm = 1100 )更具吸收性±200 cm 2 g –1)和噻吩SOA(k 375 = 0.003±0.002,SSA 375 = 0.98±0.01,⟨MAC⟩290–700 nm = 3000±500 cm 2 g –1)。相比于其他的SOA系统,MAC的报道在这项研究比那些从生物前体高和类似的高NO X人为的SOA。使用高分辨率质谱测量法对SOA分子组成进行表征,发现来自所有这三种前体的BrC中的不饱和杂环硝基产物或有机硝酸盐可能是发色团。这些发现揭示了火源杂环化合物(特别是吡咯)在夜间的氧化作用,认为这可能是BrC的来源。
更新日期:2019-02-07
中文翻译:

不饱和杂环挥发性有机化合物夜间化学过程中形成的褐碳
夜间大气处理会增强生物质燃烧烟羽中棕色碳气溶胶(BrC)的形成。杂环化合物可能是BrC的来源,杂环化合物是在燃烧生物质的烟雾中丰富的一组挥发性有机化合物(VOC)。在这里,我们研究了作为BrC来源的三种不饱和杂环化合物(吡咯,呋喃和噻吩)的硝酸根(NO 3)引发的氧化。所产生的二次有机气溶胶(SOA)在375 nm(k 375)处的折射率的虚部,在375 nm(SSA 375)处的单散射反照率和平均质量吸收系数(⟨MAC⟩290–700 nm)被报道。与呋喃和噻吩相比,NO 3吡咯的氧化具有最高的SOA产量。吡咯SOA(k 375 = 0.015±0.003,SSA = 0.86±0.01,⟨MAC⟩290–700 nm = 3400±700 cm 2 g –1)也比呋喃SOA(⟨MAC⟩290–700 nm = 1100 )更具吸收性±200 cm 2 g –1)和噻吩SOA(k 375 = 0.003±0.002,SSA 375 = 0.98±0.01,⟨MAC⟩290–700 nm = 3000±500 cm 2 g –1)。相比于其他的SOA系统,MAC的报道在这项研究比那些从生物前体高和类似的高NO X人为的SOA。使用高分辨率质谱测量法对SOA分子组成进行表征,发现来自所有这三种前体的BrC中的不饱和杂环硝基产物或有机硝酸盐可能是发色团。这些发现揭示了火源杂环化合物(特别是吡咯)在夜间的氧化作用,认为这可能是BrC的来源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号