Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Scalable production of core–shell nanoparticles by flash nanocomplexation to enhance mucosal transport for oral delivery of insulin†
Nanoscale ( IF 6.7 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1039/c7nr08047f Zhiyu He 1, 2, 3, 4, 5 , Zhijia Liu 1, 2, 3, 4, 5 , Houkuan Tian 1, 2, 3, 4, 5 , Yizong Hu 6, 7, 8, 9, 10 , Lixin Liu 1, 2, 3, 4, 5 , Kam W. Leong 1, 2, 3, 4, 5 , Hai-Quan Mao 1, 2, 3, 4, 5 , Yongming Chen 1, 2, 3, 4, 5
Nanoscale ( IF 6.7 ) Pub Date : 2018-01-25 00:00:00 , DOI: 10.1039/c7nr08047f Zhiyu He 1, 2, 3, 4, 5 , Zhijia Liu 1, 2, 3, 4, 5 , Houkuan Tian 1, 2, 3, 4, 5 , Yizong Hu 6, 7, 8, 9, 10 , Lixin Liu 1, 2, 3, 4, 5 , Kam W. Leong 1, 2, 3, 4, 5 , Hai-Quan Mao 1, 2, 3, 4, 5 , Yongming Chen 1, 2, 3, 4, 5
Affiliation
|
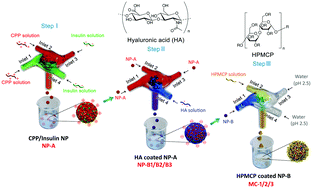
Scalable manufacturing continues to present a major barrier for clinical translation of nanotherapeutics. Methods available for fabricating protein-encapsulating nanoparticles in a scalable fashion are scarce. Protein delivery often requires multiple functionalities to be incorporated into the same vehicle. Specifically for nanoparticle-mediated oral delivery of protein therapeutics, protection in GI tract, site-specific release, facilitating transmucosal permeation, and enhancing epithelial transport are a few desirable features to be engineered into a nanoparticle system. Here we devised a sequential flash nanocomplexation (FNC) technique for the scalable production of a core–shell structured nanoparticle system by combining materials choice and particle size and structure to fulfill these functions, therefore enhancing the delivery efficiency of insulin. This method is highly effective in controlling the size, generating core–shell structure with high encapsulation efficiency (97%) and payload capacity (67%) using insulin/L-penetratin complex nanoparticles as a core coated with hyaluronic acid (HA). Both the in vitro and in vivo models confirmed that the HA coating on these core–shell nanoparticles enhanced the permeation of nanoparticles through the intestinal mucus layer and improved trans-epithelial absorption of insulin nanoparticles; and the enhancement effect was most prominent using HA with the highest average molecular weight. The insulin-loaded nanoparticles were then encapsulated into enteric microcapsules (MCs) in an FNC process to provide additional protection against the acidic environment in the stomach while allowing rapid release of insulin nanoparticles when they reach small intestine. The optimized multifunctional MCs delivered an effective glucose reduction in a Type I diabetes rat model following a single oral administration, yielding a relative bioavailability of 11% in comparison with subcutaneous injection of free-form insulin. This FNC technique is highly effective in controlling particle size and structure to improve delivery properties and function. It can be easily extended to oral delivery for other protein therapeutics.
中文翻译:

通过快速纳米复合技术可扩展生产核-壳型纳米颗粒,以增强粘膜运输以口服胰岛素†
可扩展的制造继续为纳米治疗学的临床翻译带来主要障碍。缺乏以可扩展的方式制造蛋白质包封的纳米颗粒的方法。蛋白质递送通常需要将多种功能结合到同一载体中。特别是对于纳米粒子介导的蛋白质治疗剂的口服递送,胃肠道的保护,位点特异性释放,促进透粘膜渗透和增强上皮运输是一些需要设计成纳米粒子系统的特征。在这里,我们通过结合材料选择和粒度及结构来实现这些功能,设计了一种顺序闪光纳米复合(FNC)技术,用于可扩展生产核-壳结构纳米颗粒系统,因此提高了胰岛素的输送效率。这种方法在控制尺寸方面非常有效,使用胰岛素/可以产生高封装效率(97%)和有效载荷容量(67%)的核-壳结构。以透明质酸(HA)包裹的L- penetratin复合纳米颗粒为核心。无论是在体外和体内模型证实,在这些核-壳纳米颗粒上的HA涂层增强了纳米颗粒通过肠粘液层的渗透,并改善了胰岛素纳米颗粒的跨上皮吸收。平均分子量最高的HA的增强效果最为显着。然后,以FNC工艺将载有胰岛素的纳米颗粒封装到肠溶微胶囊(MCs)中,以提供针对胃中酸性环境的额外保护,同时允许胰岛素纳米颗粒到达小肠时迅速释放。经过优化的多功能MC在单次口服后在I型糖尿病大鼠模型中有效降低了葡萄糖含量,与皮下注射游离形式胰岛素相比,其相对生物利用度为11%。这种FNC技术在控制粒径和结构以改善输送特性和功能方面非常有效。它可以很容易地扩展到其他蛋白质治疗剂的口服给药。
更新日期:2018-01-25
中文翻译:

通过快速纳米复合技术可扩展生产核-壳型纳米颗粒,以增强粘膜运输以口服胰岛素†
可扩展的制造继续为纳米治疗学的临床翻译带来主要障碍。缺乏以可扩展的方式制造蛋白质包封的纳米颗粒的方法。蛋白质递送通常需要将多种功能结合到同一载体中。特别是对于纳米粒子介导的蛋白质治疗剂的口服递送,胃肠道的保护,位点特异性释放,促进透粘膜渗透和增强上皮运输是一些需要设计成纳米粒子系统的特征。在这里,我们通过结合材料选择和粒度及结构来实现这些功能,设计了一种顺序闪光纳米复合(FNC)技术,用于可扩展生产核-壳结构纳米颗粒系统,因此提高了胰岛素的输送效率。这种方法在控制尺寸方面非常有效,使用胰岛素/可以产生高封装效率(97%)和有效载荷容量(67%)的核-壳结构。以透明质酸(HA)包裹的L- penetratin复合纳米颗粒为核心。无论是在体外和体内模型证实,在这些核-壳纳米颗粒上的HA涂层增强了纳米颗粒通过肠粘液层的渗透,并改善了胰岛素纳米颗粒的跨上皮吸收。平均分子量最高的HA的增强效果最为显着。然后,以FNC工艺将载有胰岛素的纳米颗粒封装到肠溶微胶囊(MCs)中,以提供针对胃中酸性环境的额外保护,同时允许胰岛素纳米颗粒到达小肠时迅速释放。经过优化的多功能MC在单次口服后在I型糖尿病大鼠模型中有效降低了葡萄糖含量,与皮下注射游离形式胰岛素相比,其相对生物利用度为11%。这种FNC技术在控制粒径和结构以改善输送特性和功能方面非常有效。它可以很容易地扩展到其他蛋白质治疗剂的口服给药。


































 京公网安备 11010802027423号
京公网安备 11010802027423号