当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Portal to SuFEx Click Chemistry: A Stable Fluorosulfuryl Imidazolium Salt Emerging as an “F−SO2+” Donor of Unprecedented Reactivity, Selectivity, and Scope
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-25 , DOI: 10.1002/anie.201712429 Taijie Guo 1 , Genyi Meng 1 , Xiongjie Zhan 1 , Qian Yang 2 , Tiancheng Ma 1 , Long Xu 1 , K. Barry Sharpless 1 , Jiajia Dong 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-25 , DOI: 10.1002/anie.201712429 Taijie Guo 1 , Genyi Meng 1 , Xiongjie Zhan 1 , Qian Yang 2 , Tiancheng Ma 1 , Long Xu 1 , K. Barry Sharpless 1 , Jiajia Dong 1
Affiliation
|
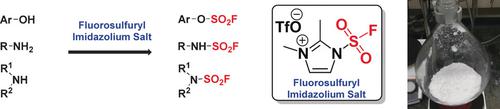
Sulfuryl fluoride, SO2F2, has been found to derivatize phenols in all kinds of environments, even those in highly functional molecules. We now report that a solid fluorosulfuryl imidazolium triflate salt delivers the same “F−SO2+” fragment to Nu−H acceptor groups in the substrates. However, this triflate salt is a far more reactive fluorosulfurylating agent than SO2F2 and displays selectivity preferences of its own. Moreover, the new azolium triflate reagent reacts once with primary amines and anilines before the reaction stops. On the other hand, with triethylamine and two equivalents of the “F−SO2+” donor present, it proceeds on to the bis(fluorosulfuryl)imides in good yield—two important conversions that we have never seen with sulfuryl fluoride as the electrophile.
中文翻译:

SuFEx Click化学的新门户:稳定的氟磺酰基咪唑鎓盐,以前所未有的反应性,选择性和范围成为“ F-SO2 +”供体
已经发现,硫酰氟SO 2 F 2在各种环境中,甚至在高功能分子中,都可以使苯酚衍生化。现在,我们报告固体三氟甲磺酸氟磺酰基咪唑鎓盐向底物中的Nu-H受体基团传递相同的“ F-SO 2 + ”片段。但是,这种三氟甲磺酸盐是一种比SO 2 F 2更具活性的氟磺化剂,并显示出其自身的选择性偏好。而且,在停止反应之前,新的三氟甲磺酸偶氮鎓试剂与伯胺和苯胺反应一次。另一方面,用三乙胺和两当量的“ F-SO 2 +“供体存在,它以良好的收率继续进行双(氟硫磺酰基)酰亚胺的转化-这是我们从未见过的两个重要的转化反应,我们将磺酰氟用作亲电试剂。
更新日期:2018-01-25
中文翻译:

SuFEx Click化学的新门户:稳定的氟磺酰基咪唑鎓盐,以前所未有的反应性,选择性和范围成为“ F-SO2 +”供体
已经发现,硫酰氟SO 2 F 2在各种环境中,甚至在高功能分子中,都可以使苯酚衍生化。现在,我们报告固体三氟甲磺酸氟磺酰基咪唑鎓盐向底物中的Nu-H受体基团传递相同的“ F-SO 2 + ”片段。但是,这种三氟甲磺酸盐是一种比SO 2 F 2更具活性的氟磺化剂,并显示出其自身的选择性偏好。而且,在停止反应之前,新的三氟甲磺酸偶氮鎓试剂与伯胺和苯胺反应一次。另一方面,用三乙胺和两当量的“ F-SO 2 +“供体存在,它以良好的收率继续进行双(氟硫磺酰基)酰亚胺的转化-这是我们从未见过的两个重要的转化反应,我们将磺酰氟用作亲电试剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号