当前位置:
X-MOL 学术
›
Prog. Solid State Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water's phase diagram: From the notion of thermodynamics to hydrogen-bond cooperativity
Progress in Solid State Chemistry ( IF 9.1 ) Pub Date : 2015-09-01 , DOI: 10.1016/j.progsolidstchem.2015.03.001 Xi Zhang , Peng Sun , Tingting Yan , Yongli Huang , Zengsheng Ma , Bo Zou , Weitao Zheng , Ji Zhou , Yinyan Gong , Chang Q. Sun
Progress in Solid State Chemistry ( IF 9.1 ) Pub Date : 2015-09-01 , DOI: 10.1016/j.progsolidstchem.2015.03.001 Xi Zhang , Peng Sun , Tingting Yan , Yongli Huang , Zengsheng Ma , Bo Zou , Weitao Zheng , Ji Zhou , Yinyan Gong , Chang Q. Sun
|
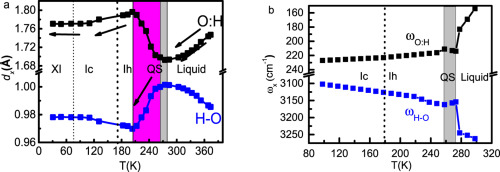
Abstract This presentation features recent progress in understanding the phase diagram of water and ice from the perspective of hydrogen bond (O:H–O) cooperative relaxation with focus on how the segmental length and the containing angle of the O:H–O bond change with mechanical compression and thermal excitation. By interplaying theoretical predictions, numerical computations, and phonon spectrometrics, we firstly examined the relaxation dynamics of O:H–O bond segmental length and phonon stiffness of: i) liquid water at 300 K and ice at 80 K as a function of pressure, ii) liquid water cooling from 350 K to 80 K under the ambient pressure, iii) mechanical freezing of the ambient water under compression up to 1.83 GPa, and, iv) liquid water heating from 253 to 753 K under 30 MPa pressure. Observations allow us to classify the TC(P) phase boundaries of water and ice into four types according to their slopes and then formulate them in terms of hydrogen bond relaxation in segmental length and containing angle. Observations reinforce the essentiality and effectiveness of hydrogen bond notion in dictating the unusual behavior of water and ice and clarify the bonding dynamics during phase transition, which is beyond the scope of classical thermodynamics.
中文翻译:

水的相图:从热力学概念到氢键协同性
摘要 本报告重点介绍了从氢键 (O:H-O) 协同弛豫角度理解水和冰相图的最新进展,重点关注 O:H-O 键的链段长度和夹角如何变化具有机械压缩和热激发。通过理论预测、数值计算和声子光谱学的相互作用,我们首先检查了以下物质的 O:H-O 键段长度和声子刚度的弛豫动力学:i) 作为压力函数的 300 K 液态水和 80 K 冰, ii) 在环境压力下将液态水从 350 K 冷却到 80 K,iii) 在高达 1.83 GPa 的压缩下机械冷冻环境水,以及,iv) 在 30 MPa 压力下将液态水从 253 K 加热到 753 K。观测使我们可以根据斜率将水和冰的 TC(P) 相边界分为四种类型,然后根据段长和包含角的氢键弛豫来表示它们。观察加强了氢键概念在决定水和冰的异常行为方面的重要性和有效性,并阐明了相变过程中的键合动力学,这超出了经典热力学的范围。
更新日期:2015-09-01
中文翻译:

水的相图:从热力学概念到氢键协同性
摘要 本报告重点介绍了从氢键 (O:H-O) 协同弛豫角度理解水和冰相图的最新进展,重点关注 O:H-O 键的链段长度和夹角如何变化具有机械压缩和热激发。通过理论预测、数值计算和声子光谱学的相互作用,我们首先检查了以下物质的 O:H-O 键段长度和声子刚度的弛豫动力学:i) 作为压力函数的 300 K 液态水和 80 K 冰, ii) 在环境压力下将液态水从 350 K 冷却到 80 K,iii) 在高达 1.83 GPa 的压缩下机械冷冻环境水,以及,iv) 在 30 MPa 压力下将液态水从 253 K 加热到 753 K。观测使我们可以根据斜率将水和冰的 TC(P) 相边界分为四种类型,然后根据段长和包含角的氢键弛豫来表示它们。观察加强了氢键概念在决定水和冰的异常行为方面的重要性和有效性,并阐明了相变过程中的键合动力学,这超出了经典热力学的范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号