Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.tetlet.2017.09.025 Arun K. Ghosh , Emilio L. Cárdenas , Margherita Brindisi
|
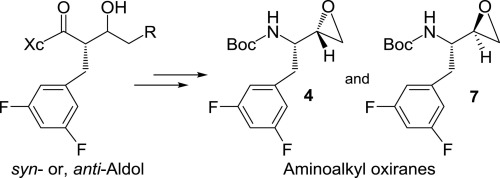
Enantioselective syntheses of tert-butyl ((S)-2-(3,5-difluorophenyl)-1-((S)-oxiran-2-yl)ethyl)carbamate and ((S)-2-(3,5-difluorophenyl)-1-((R)-oxiran-2-yl)ethyl)carbamate are described. We utilized asymmetric syn- and anti-aldol reactions to set both stereogenic centers. We investigated ester-derived Ti-enolate aldol reactions as well as Evans’ diastereoselective syn-aldol reaction for these syntheses. We have converted optically active ((S)-2-(3,5-difluorophenyl)-1-((S)-oxiran-2-yl)ethyl)carbamate to a potent β-secretase inhibitor.
中文翻译:

叔丁基-2-(3,5-二氟苯基)-1-环氧乙烷-2-基)乙基氨基甲酸酯的高度立体选择性的不对称羟醛路径:新型蛋白酶抑制剂的结构单元
的对映选择性合成叔丁基((小号)-2-(3,5-二氟苯基)-1 - ((小号) -环氧乙烷-2-基)乙基)氨基甲酸叔丁酯和((小号)-2-(3,5-描述了二氟苯基)-1-((R)-环氧乙烷-2-基)乙基)氨基甲酸酯。我们利用不对称的顺和反羟醛反应来设定两个立体异构中心。对于这些合成,我们研究了酯衍生的钛-烯醇醛醇醛缩合反应以及埃文斯的非对映选择性合成-醇醛缩合反应。我们已经将旋光性((S)-2-(3,5-二氟苯基)-1-((S)-环氧乙烷-2-基)乙基)氨基甲酸酯转变为有效的β-分泌酶抑制剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号