当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoparticle Properties for Delivery to Cartilage: The Implications of Disease State, Synovial Fluid, and Off-Target Uptake
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-07-01 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00484 Shannon Brown 1 , Jake Pistiner 1 , Isaac M. Adjei 1 , Blanka Sharma 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-07-01 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00484 Shannon Brown 1 , Jake Pistiner 1 , Isaac M. Adjei 1 , Blanka Sharma 1
Affiliation
|
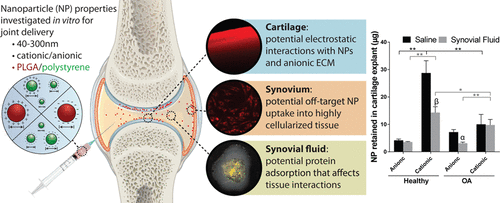
A major hurdle limiting the ability to treat and cure osteoarthritis, a common and debilitating disease, is rapid joint clearance and limited cartilage targeting of intra-articular therapies. Nanoscale drug carriers have the potential to improve therapeutic targeting and retention in the joint after direct injection; however, there still lacks a fundamental understanding of how the physicochemical properties of nanoparticles (NPs) influence localization to the degenerating cartilage and how joint conditions such as disease state and synovial fluid impact NP biodistribution. The goal of this study was to assess how physicochemical properties of NPs influence their interactions with joint tissues and, ultimately, cartilage localization. Ex vivo models of joint tissues were used to study how poly(lactide-co-glycolide) (PLGA) and polystyrene (PS) NP size, charge, and surface chemistry influence cartilage retention under normal and disease-mimicking conditions. Of the particles investigated, PLGA NPs surface-modified with a quaternary ammonium cation had the greatest retention within cartilage explants; however, retention was diminished 2- to 2.9-fold in arthritic tissue and in the presence of synovial fluid. Interactions with synovial fluid induced changes to NP surface properties and colloidal stability in vitro. The impact of NP charge on “off-target” synoviocyte uptake was also dependent on synovial fluid interactions. The results suggest that the design of nanocarriers for targeted drug delivery within the joint cannot be based on a single parameter such as zeta potential or size, and that the fate of injected delivery systems will likely be influenced by the disease state of the joint and the presence of synovial fluid.
中文翻译:

纳米性质传递到软骨:疾病状态,滑液和脱靶摄取的影响。
限制关节内治疗的快速关节清除率和有限的软骨靶向性是限制治疗和治疗骨关节炎(一种常见且使人衰弱的疾病)的能力的主要障碍。纳米级药物载体在直接注射后具有改善治疗靶向性和保留关节的潜力。但是,对于纳米粒子(NPs)的物理化学特性如何影响变性软骨的定位以及诸如疾病状态和滑液等关节疾病如何影响NP生物分布,仍然缺乏基本的了解。这项研究的目的是评估NP的理化特性如何影响其与关节组织的相互作用,并最终影响软骨的定位。关节组织的离体模型用于研究聚丙交酯-共-glycolide)(PLGA)和聚苯乙烯(PS)NP大小,电荷和表面化学的影响软骨保持正常和疾病-模拟条件下进行。在所研究的颗粒中,经季铵阳离子表面改性的PLGA NP在软骨外植体中的保留最大。然而,在滑液存在的情况下,关节炎组织中的保留减少了2到2.9倍。与滑液的相互作用引起体外NP表面性质和胶体稳定性的改变。NP电荷对“脱靶”滑膜细胞摄取的影响还取决于滑液相互作用。结果表明,针对关节内靶向药物递送的纳米载体的设计不能基于诸如zeta电位或大小之类的单一参数,并且注射递送系统的命运将很可能受到关节疾病状态和血管内皮生长的影响。滑液的存在。
更新日期:2017-07-01
中文翻译:

纳米性质传递到软骨:疾病状态,滑液和脱靶摄取的影响。
限制关节内治疗的快速关节清除率和有限的软骨靶向性是限制治疗和治疗骨关节炎(一种常见且使人衰弱的疾病)的能力的主要障碍。纳米级药物载体在直接注射后具有改善治疗靶向性和保留关节的潜力。但是,对于纳米粒子(NPs)的物理化学特性如何影响变性软骨的定位以及诸如疾病状态和滑液等关节疾病如何影响NP生物分布,仍然缺乏基本的了解。这项研究的目的是评估NP的理化特性如何影响其与关节组织的相互作用,并最终影响软骨的定位。关节组织的离体模型用于研究聚丙交酯-共-glycolide)(PLGA)和聚苯乙烯(PS)NP大小,电荷和表面化学的影响软骨保持正常和疾病-模拟条件下进行。在所研究的颗粒中,经季铵阳离子表面改性的PLGA NP在软骨外植体中的保留最大。然而,在滑液存在的情况下,关节炎组织中的保留减少了2到2.9倍。与滑液的相互作用引起体外NP表面性质和胶体稳定性的改变。NP电荷对“脱靶”滑膜细胞摄取的影响还取决于滑液相互作用。结果表明,针对关节内靶向药物递送的纳米载体的设计不能基于诸如zeta电位或大小之类的单一参数,并且注射递送系统的命运将很可能受到关节疾病状态和血管内皮生长的影响。滑液的存在。










































 京公网安备 11010802027423号
京公网安备 11010802027423号