当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight into the Reactivity of Chlorine-Derived Radicals in the Aqueous-Phase UV–Chlorine Advanced Oxidation Process: Quantum Mechanical Calculations
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2017-05-25 00:00:00 , DOI: 10.1021/acs.est.7b00507 Daisuke Minakata 1 , Divya Kamath 1 , Shaye Maetzold 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2017-05-25 00:00:00 , DOI: 10.1021/acs.est.7b00507 Daisuke Minakata 1 , Divya Kamath 1 , Shaye Maetzold 1
Affiliation
|
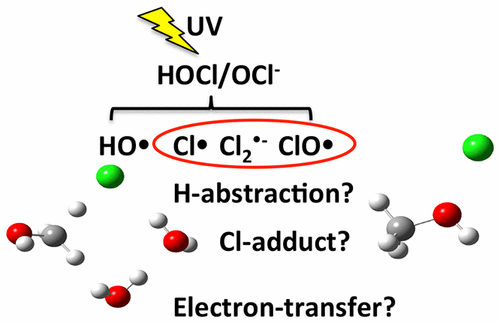
The combined ultraviolet (UV) and free chlorine (UV–chlorine) advanced oxidation process that produces highly reactive hydroxyl radicals (HO•) and chlorine radicals (Cl•) is an attractive alternative to UV alone or chlorination for disinfection because of the destruction of a wide variety of organic compounds. However, concerns about the potential formation of chlorinated transformation products require an understanding of the radical-induced elementary reaction mechanisms and their reaction-rate constants. While many studies have revealed the reactivity of oxygenated radicals, the reaction mechanisms of chlorine-derived radicals have not been elucidated due to the data scarcity and discrepancies among experimental observations. We found a linear free-energy relationship quantum mechanically calculated free energies of reaction and the literature-reported experimentally measured reaction rate constants, kexp, for 22 chlorine-derived inorganic radical reactions in the UV–chlorine process. This relationship highlights the discrepancy among literature-reported rate constants and aids in the determination of the rate constant using quantum mechanical calculations. We also found linear correlations between the theoretically calculated free energies of activation and kexp for 31 reactions of Cl• with organic compounds. The correlation suggests that H-abstraction and Cl-adduct formation are the major reaction mechanisms. This is the first comprehensive study on chlorine-derived radical reactions, and it provides mechanistic insight into the reaction mechanisms for the development of an elementary reaction-based kinetic model.
中文翻译:

机械了解水相UV-氯高级氧化过程中氯基自由基的反应性:量子力学计算
紫外线(UV)和游离氯(UV-氯)的组合高级氧化工艺可产生高反应性的羟基自由基(HO •)和氯自由基(Cl •)是一种很有吸引力的替代品,因为它会破坏多种有机化合物,因此可以单独使用UV或氯化消毒。然而,对氯化转化产物可能形成的担忧需要了解自由基引发的基本反应机理及其反应速率常数。尽管许多研究已经揭示了含氧自由基的反应性,但由于数据缺乏和实验观察之间的差异,氯源自由基的反应机理尚未阐明。我们发现了线性自由能关系,量子力学计算了反应的自由能,文献报道了实验测得的反应速率常数,k exp,用于UV-氯过程中的22个氯衍生的无机自由基反应。这种关系突出了文献报道的速率常数之间的差异,并有助于使用量子力学计算确定速率常数。我们还发现,理论计算的活化自由能与Cl •与有机化合物的31个反应的k exp之间存在线性关系。相关性表明,H吸收和Cl加合物的形成是主要的反应机理。这是对氯衍生的自由基反应的首次全面研究,它为开发基于基本反应的动力学模型的反应机理提供了机械方面的见识。
更新日期:2017-06-10
中文翻译:

机械了解水相UV-氯高级氧化过程中氯基自由基的反应性:量子力学计算
紫外线(UV)和游离氯(UV-氯)的组合高级氧化工艺可产生高反应性的羟基自由基(HO •)和氯自由基(Cl •)是一种很有吸引力的替代品,因为它会破坏多种有机化合物,因此可以单独使用UV或氯化消毒。然而,对氯化转化产物可能形成的担忧需要了解自由基引发的基本反应机理及其反应速率常数。尽管许多研究已经揭示了含氧自由基的反应性,但由于数据缺乏和实验观察之间的差异,氯源自由基的反应机理尚未阐明。我们发现了线性自由能关系,量子力学计算了反应的自由能,文献报道了实验测得的反应速率常数,k exp,用于UV-氯过程中的22个氯衍生的无机自由基反应。这种关系突出了文献报道的速率常数之间的差异,并有助于使用量子力学计算确定速率常数。我们还发现,理论计算的活化自由能与Cl •与有机化合物的31个反应的k exp之间存在线性关系。相关性表明,H吸收和Cl加合物的形成是主要的反应机理。这是对氯衍生的自由基反应的首次全面研究,它为开发基于基本反应的动力学模型的反应机理提供了机械方面的见识。

































 京公网安备 11010802027423号
京公网安备 11010802027423号