当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total synthesis of (−)-securingine G
Chemical Communications ( IF 4.9 ) Pub Date : 2024-05-23 , DOI: 10.1039/d4cc01075b Taesik Youn 1 , Taewan Kim 1 , Sunkyu Han 1
Chemical Communications ( IF 4.9 ) Pub Date : 2024-05-23 , DOI: 10.1039/d4cc01075b Taesik Youn 1 , Taewan Kim 1 , Sunkyu Han 1
Affiliation
|
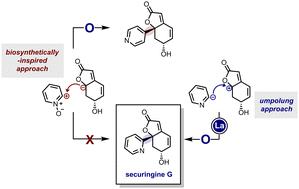
In this study, we present the first total synthesis of (−)-securingine G. Diverging from the proposed biosynthetic pathway, our approach involves the addition of nucleophilic pyridine anion species to the electrophilic menisdaurilide congener. Crucially, incorporating a weakly basic yet nucleophilic tri(2-pyridinyl)lanthanum complex proved essential to circumvent undesired base-mediated pathways during the key coupling reaction. Notably, we introduce n-Bu3La·5LiCl as a new exchange reagent, facilitating efficient halide/lanthanum exchange of (hetero)aryl halides.
中文翻译:

(−)-亚叶碱G的全合成
在这项研究中,我们首次全合成了(−)-securingine G。与所提出的生物合成途径不同,我们的方法涉及将亲核吡啶阴离子添加到亲电menisdaurilide同系物中。至关重要的是,事实证明,掺入弱碱性但亲核的三(2-吡啶基)镧配合物对于在关键偶联反应过程中规避不需要的碱介导途径至关重要。值得注意的是,我们引入了 n-Bu 3 La·5LiCl 作为一种新的交换试剂,促进了(杂)芳基卤化物的有效卤化物/镧交换。
更新日期:2024-05-23
中文翻译:

(−)-亚叶碱G的全合成
在这项研究中,我们首次全合成了(−)-securingine G。与所提出的生物合成途径不同,我们的方法涉及将亲核吡啶阴离子添加到亲电menisdaurilide同系物中。至关重要的是,事实证明,掺入弱碱性但亲核的三(2-吡啶基)镧配合物对于在关键偶联反应过程中规避不需要的碱介导途径至关重要。值得注意的是,我们引入了 n-Bu 3 La·5LiCl 作为一种新的交换试剂,促进了(杂)芳基卤化物的有效卤化物/镧交换。

































 京公网安备 11010802027423号
京公网安备 11010802027423号