当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A reversible self-assembled molecular layer for lithium metal batteries with high energy/power densities at ultra-low temperatures
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4ee01298d Weili Zhang 1, 2 , Yang Lu 1 , Qingbin Cao 1 , Hao Liu 1 , Qingqing Feng 2 , Pan Zhou 1 , Yingchun Xia 1 , Wenhui Hou 1 , Shuaishuai Yan 1 , Kai Liu 1, 2, 3
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4ee01298d Weili Zhang 1, 2 , Yang Lu 1 , Qingbin Cao 1 , Hao Liu 1 , Qingqing Feng 2 , Pan Zhou 1 , Yingchun Xia 1 , Wenhui Hou 1 , Shuaishuai Yan 1 , Kai Liu 1, 2, 3
Affiliation
|
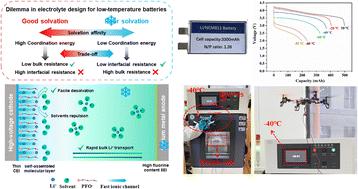
Electrolytes for low temperature, high energy lithium metal batteries are expected to possess both fast Li+ transfer in the bulk electrolytes (low bulk resistance) and a fast Li+ de-solvation process at the electrode/electrolyte interface (low interfacial resistance). However, the nature of the solvent determines that the two always stand at either ends of the balance, and conventional electrolyte designs have to make a compromise to favor one at the expense of the other. Here, we address this irreconcilable dilemma using an “electric-field assisted self-assembly layer”, i.e. sodium perfluorooctanoate (NaPFO). Driven by the bias-potential, PFO− anions dissolved in the electrolyte could reversibly self-assemble into a dense and ordered molecular layer at the cathode/electrolyte interface, which could protect the electrolyte from anodic degradation due to the high voltage and enable the stable cycling of LiNi0.8Mn0.1Co0.1O2 (NMC811) in ether-based electrolyte with an ultra-low freeing point. More importantly, the self-assembly of the PFO− layer could aid the Li+ de-solvation behavior at the cathode/electrolyte interface, thus significantly lowering the interfacial resistance. Thus, with this reversible self-assembly layer on the cathode surface, low bulk resistance and low interfacial resistance are simultaneously achieved. Consequently, the tetrahydrofuran (THF)-based electrolyte containing NaPFO is applied to practical Li‖NMC811 pouch cells and achieves an unprecedented energy density of 122 W h kg−1 (except taps and packing foil, same hereafter) at −85 °C, the highest reported power density of 318 W kg−1 at a high current density of 4.1 mA cm−2 at −60 °C, and can be recharged with stable performance (175 W h kg−1 at 20th cycles) at −40 °C. This electric-field assisted self-assembly layer enables fine tuning of the micro-environment at the cathode–electrolyte interface, and provides a new design concept for the electrolyte of ultra-low temperature high voltage lithium-metal batteries.
中文翻译:

超低温下具有高能量/功率密度的锂金属电池的可逆自组装分子层
用于低温、高能锂金属电池的电解质预计在本体电解质中具有快速的Li + 转移(低体电阻)和快速的Li + 去溶剂化过程。电极/电解质界面(低界面电阻)。然而,溶剂的性质决定了两者总是处于天平的两端,传统的电解质设计必须做出妥协,以牺牲另一种为代价,以偏向其中一种。在这里,我们使用“电场辅助自组装层”,即全氟辛酸钠(NaPFO)来解决这个不可调和的困境。在偏压驱动下,溶解在电解液中的 PFO − 阴离子可以在阴极/电解液界面可逆地自组装成致密且有序的分子层,从而保护电解液免受阳极降解。高电压,使 LiNi 0.8 Mn 0.1 Co 0.1 O 2 (NMC811) 在醚基电解液中稳定循环超低自由点。更重要的是,PFO − 层的自组装有助于正极/电解质界面的 Li + 去溶剂化行为,从而显着降低界面电阻。因此,通过阴极表面上的这种可逆自组装层,可以同时实现低体电阻和低界面电阻。因此,含有NaPFO的四氢呋喃(THF)基电解质被应用于实用的Li‖NMC811软包电池,并在122 W h kg −1 (除了抽头和包装箔,下同)达到了前所未有的能量密度。 -85 °C,在 4 的高电流密度下,报道的最高功率密度为 318 W kg −1 。-60 °C 时为 1 mA cm −2 ,-40 °C 时可再充电且性能稳定(第 20 次循环时为 175 W h kg −1 )。这种电场辅助自组装层能够微调正极-电解质界面的微环境,为超低温高压锂金属电池电解质提供了新的设计理念。
更新日期:2024-05-22
中文翻译:

超低温下具有高能量/功率密度的锂金属电池的可逆自组装分子层
用于低温、高能锂金属电池的电解质预计在本体电解质中具有快速的Li + 转移(低体电阻)和快速的Li + 去溶剂化过程。电极/电解质界面(低界面电阻)。然而,溶剂的性质决定了两者总是处于天平的两端,传统的电解质设计必须做出妥协,以牺牲另一种为代价,以偏向其中一种。在这里,我们使用“电场辅助自组装层”,即全氟辛酸钠(NaPFO)来解决这个不可调和的困境。在偏压驱动下,溶解在电解液中的 PFO − 阴离子可以在阴极/电解液界面可逆地自组装成致密且有序的分子层,从而保护电解液免受阳极降解。高电压,使 LiNi 0.8 Mn 0.1 Co 0.1 O 2 (NMC811) 在醚基电解液中稳定循环超低自由点。更重要的是,PFO − 层的自组装有助于正极/电解质界面的 Li + 去溶剂化行为,从而显着降低界面电阻。因此,通过阴极表面上的这种可逆自组装层,可以同时实现低体电阻和低界面电阻。因此,含有NaPFO的四氢呋喃(THF)基电解质被应用于实用的Li‖NMC811软包电池,并在122 W h kg −1 (除了抽头和包装箔,下同)达到了前所未有的能量密度。 -85 °C,在 4 的高电流密度下,报道的最高功率密度为 318 W kg −1 。-60 °C 时为 1 mA cm −2 ,-40 °C 时可再充电且性能稳定(第 20 次循环时为 175 W h kg −1 )。这种电场辅助自组装层能够微调正极-电解质界面的微环境,为超低温高压锂金属电池电解质提供了新的设计理念。

































 京公网安备 11010802027423号
京公网安备 11010802027423号