当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acridine photocatalysis enables tricomponent direct decarboxylative amine construction
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02356k Xianwei Sui 1 , Hang T. Dang 1 , Arka Porey 1 , Ramon Trevino 1 , Arko Das 1 , Seth O. Fremin 1 , William B. Hughes 1 , William T. Thompson 1 , Shree Krishna Dhakal 1 , Hadi D. Arman 1 , Oleg V. Larionov 1
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02356k Xianwei Sui 1 , Hang T. Dang 1 , Arka Porey 1 , Ramon Trevino 1 , Arko Das 1 , Seth O. Fremin 1 , William B. Hughes 1 , William T. Thompson 1 , Shree Krishna Dhakal 1 , Hadi D. Arman 1 , Oleg V. Larionov 1
Affiliation
|
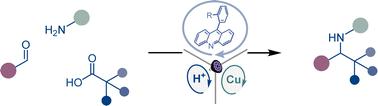
Amines are centrally important motifs in medicinal chemistry and biochemistry, and indispensable intermediates and linchpins in organic synthesis. Despite their cross-disciplinary prominence, synthetic access to amine continues to rely on two-electron approaches based on reductions and additions of organometallic reagents, limiting their accessible chemical space and necessitating stepwise preassembly of synthetic precursors. We report herein a homogeneous photocatalytic tricomponent decarboxylative radical-mediated amine construction that enables modular access to α-branched secondary amines directly from the broad and structurally diverse chemical space of carboxylic acids in a tricomponent reaction with aldehydes and aromatic amines. Our studies reveal the key role of acridine photocatalysis acting in concert with copper and Brønsted acid catalytic processes in facilitating the previously inaccessible homogeneous photocatalytic reaction and provide a streamlined segue to a wide range of amines and nonproteinogenic α-amino acids.
中文翻译:

吖啶光催化实现三组分直接脱羧胺构建
胺是药物化学和生物化学中的核心重要基序,也是有机合成中不可或缺的中间体和关键。尽管它们具有跨学科的突出地位,但胺的合成仍然依赖于基于有机金属试剂的减少和添加的双电子方法,限制了它们的可利用化学空间,并且需要逐步预组装合成前体。我们在此报道了一种均相光催化三组分脱羧自由基介导的胺结构,该结构能够在与醛和芳香胺的三组分反应中直接从羧酸的广泛且结构多样的化学空间模块化获得α-支化仲胺。我们的研究揭示了吖啶光催化与铜和布朗斯台德酸催化过程协同作用在促进以前难以实现的均相光催化反应中的关键作用,并为各种胺和非蛋白性α-氨基酸提供了简化的序列。
更新日期:2024-05-22
中文翻译:

吖啶光催化实现三组分直接脱羧胺构建
胺是药物化学和生物化学中的核心重要基序,也是有机合成中不可或缺的中间体和关键。尽管它们具有跨学科的突出地位,但胺的合成仍然依赖于基于有机金属试剂的减少和添加的双电子方法,限制了它们的可利用化学空间,并且需要逐步预组装合成前体。我们在此报道了一种均相光催化三组分脱羧自由基介导的胺结构,该结构能够在与醛和芳香胺的三组分反应中直接从羧酸的广泛且结构多样的化学空间模块化获得α-支化仲胺。我们的研究揭示了吖啶光催化与铜和布朗斯台德酸催化过程协同作用在促进以前难以实现的均相光催化反应中的关键作用,并为各种胺和非蛋白性α-氨基酸提供了简化的序列。

































 京公网安备 11010802027423号
京公网安备 11010802027423号