当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
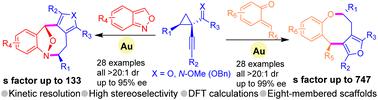
Kinetic resolution of 1-(1-alkynyl)cyclopropyl ketones via gold-catalyzed divergent (4 + 4) cycloadditions: stereoselective access to furan fused eight-membered heterocycles
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02763a Xunhua Wang 1 , Ruifeng Lv 1 , Xiaoxun Li 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02763a Xunhua Wang 1 , Ruifeng Lv 1 , Xiaoxun Li 1, 2
Affiliation
|
Chiral eight-membered heterocycles comprise a diverse array of natural products and bioactive compounds, yet accessing them poses significant challenges. Here we report a gold-catalyzed stereoselective (4 + 4) cycloaddition as a reliable and divergent strategy, enabling readily accessible precursors (anthranils and ortho-quinone methides) to be intercepted by in situ generated gold-furyl 1,4-dipoles, delivering previously inaccessible chiral furan/pyrrole-containing eight-membered heterocycles with good results (56 examples, all >20 : 1 dr, up to 99% ee). Moreover, we achieve a remarkably efficient kinetic resolution (KR) process (s factor up to 747). The scale-up synthesis and diversified transformations of cycloadducts highlight the synthetic potential of this protocol. Computational calculations provide an in-depth understanding of the stereoselective cycloaddition process.
中文翻译:

通过金催化发散 (4 + 4) 环加成反应动力学拆分 1-(1-炔基)环丙基酮:立体选择性获得呋喃稠合八元杂环
手性八元杂环由多种天然产物和生物活性化合物组成,但获取它们面临着巨大的挑战。在这里,我们报告了金催化的立体选择性(4 + 4)环加成作为一种可靠且发散的策略,使易于获得的前体(邻氨基苯甲醚和邻醌甲基化物)能够被原位生成的金-呋喃基1,4-偶极子拦截,从而提供以前无法获得的含手性呋喃/吡咯的八元杂环,取得了良好的结果(56 个例子,全部 >20 : 1 dr,高达 99% ee)。此外,我们实现了非常高效的动力学拆分 (KR) 过程(s 因子高达 747)。环加合物的放大合成和多样化转化凸显了该方案的合成潜力。计算提供了对立体选择性环加成过程的深入理解。
更新日期:2024-05-22
中文翻译:

通过金催化发散 (4 + 4) 环加成反应动力学拆分 1-(1-炔基)环丙基酮:立体选择性获得呋喃稠合八元杂环
手性八元杂环由多种天然产物和生物活性化合物组成,但获取它们面临着巨大的挑战。在这里,我们报告了金催化的立体选择性(4 + 4)环加成作为一种可靠且发散的策略,使易于获得的前体(邻氨基苯甲醚和邻醌甲基化物)能够被原位生成的金-呋喃基1,4-偶极子拦截,从而提供以前无法获得的含手性呋喃/吡咯的八元杂环,取得了良好的结果(56 个例子,全部 >20 : 1 dr,高达 99% ee)。此外,我们实现了非常高效的动力学拆分 (KR) 过程(s 因子高达 747)。环加合物的放大合成和多样化转化凸显了该方案的合成潜力。计算提供了对立体选择性环加成过程的深入理解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号