当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-selective S-gem-difluoroallylation of unprotected peptides with 3,3-difluoroallyl sulfonium salts
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02681k Jin-Xiu Ren 1 , Minqi Zhou 1 , Xiao-Tian Feng 1 , Hai-Yang Zhao 1 , Xia-Ping Fu 1 , Xingang Zhang 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02681k Jin-Xiu Ren 1 , Minqi Zhou 1 , Xiao-Tian Feng 1 , Hai-Yang Zhao 1 , Xia-Ping Fu 1 , Xingang Zhang 1, 2
Affiliation
|
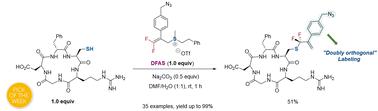
Bench-stable 3,3-difluoroallyl sulfonium salts (DFASs), featuring tunable activity and their editable C-β and gem-difluoroallyl group, proved to be versatile fluoroalkylating reagents for site-selective S-gem-difluoroallylation of cysteine residues in unprotected peptides. The reaction proceeds with high efficiency under mild conditions (ambient temperature and aqueous and weak basic conditions). Various protected/unprotected peptides, especially bioactive peptides, are site-selectively S-gem-difluoroallylated. The newly added gem-difluoroallyl group and other functional groups derived from C-β of DFASs are poised for ligation with bio-functional groups through click and radical chemistry. This stepwise “doubly orthogonal” modification of peptides enables the construction of bioconjugates with enhanced complexity and functionality. This proof of principle is successfully applied to construct a peptide–saccharide–biotin chimeric bioconjugate, indicating its great potential application in medicinal chemistry and chemical biology.
中文翻译:

用 3,3-二氟烯丙基锍盐对未受保护的肽进行位点选择性 S-gem-二氟烯丙基化
实验室稳定的 3,3-二氟烯丙基锍盐 (DFAS) 具有可调节的活性及其可编辑的 C-β 和偕二氟烯丙基基团,被证明是用于对未受保护的肽中的半胱氨酸残基进行位点选择性 S-gem-二氟烯丙基化的通用氟烷基化试剂。该反应在温和条件(环境温度、水相和弱碱性条件)下高效进行。各种受保护/未受保护的肽,尤其是生物活性肽,被位点选择性地 S-gem-二氟烯丙基化。新添加的偕二氟烯丙基和源自 DFAS 的 C-β 的其他官能团准备通过点击和自由基化学与生物官能团连接。这种肽的逐步“双正交”修饰使得能够构建具有增强的复杂性和功能性的生物缀合物。这一原理证明已成功应用于构建肽-糖-生物素嵌合生物缀合物,表明其在药物化学和化学生物学中的巨大应用潜力。
更新日期:2024-05-22
中文翻译:

用 3,3-二氟烯丙基锍盐对未受保护的肽进行位点选择性 S-gem-二氟烯丙基化
实验室稳定的 3,3-二氟烯丙基锍盐 (DFAS) 具有可调节的活性及其可编辑的 C-β 和偕二氟烯丙基基团,被证明是用于对未受保护的肽中的半胱氨酸残基进行位点选择性 S-gem-二氟烯丙基化的通用氟烷基化试剂。该反应在温和条件(环境温度、水相和弱碱性条件)下高效进行。各种受保护/未受保护的肽,尤其是生物活性肽,被位点选择性地 S-gem-二氟烯丙基化。新添加的偕二氟烯丙基和源自 DFAS 的 C-β 的其他官能团准备通过点击和自由基化学与生物官能团连接。这种肽的逐步“双正交”修饰使得能够构建具有增强的复杂性和功能性的生物缀合物。这一原理证明已成功应用于构建肽-糖-生物素嵌合生物缀合物,表明其在药物化学和化学生物学中的巨大应用潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号