当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Revisiting the reaction pathways for phospholipid hydrolysis catalyzed by phospholipase A2 with QM/MM methods
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02315c Alexandre V. Pinto 1 , Pedro Ferreira 1 , Ana V. Cunha 2 , Remco W. A. Havenith 3, 4 , Alexandre L. Magalhães 1 , Maria J. Ramos 1 , Pedro A. Fernandes 1
Chemical Science ( IF 8.4 ) Pub Date : 2024-05-22 , DOI: 10.1039/d4sc02315c Alexandre V. Pinto 1 , Pedro Ferreira 1 , Ana V. Cunha 2 , Remco W. A. Havenith 3, 4 , Alexandre L. Magalhães 1 , Maria J. Ramos 1 , Pedro A. Fernandes 1
Affiliation
|
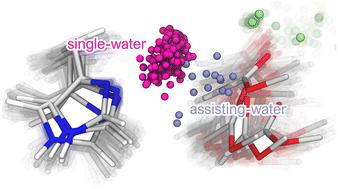
Secreted phospholipase A2 (sPLA2) is a Ca2+-dependent, widely distributed enzyme superfamily in almost all mammalian tissues and bacteria. It is also a critical component of the venom of nearly all snakes, as well as many invertebrate species. In non-venomous contexts, sPLA2 assumes significance in cellular signaling pathways by binding cell membranes and catalyzing ester bond hydrolysis at the sn-2 position of phospholipids. Elevated levels of GIIA sPLA2 have been detected in the synovial fluid of arthritis patients, where it exhibits a pro-inflammatory function. Consequently, identifying sPLA2 inhibitors holds promise for creating highly effective pharmaceutical treatments. Beyond arthritis, the similarities among GIIA sPLA2s offer an opportunity for developing treatments against snakebite envenoming, the deadliest neglected tropical disease. Despite decades of study, the details of PLA2 membrane-binding, substrate-binding, and reaction mechanism remain elusive, demanding a comprehensive understanding of the sPLA2 catalytic mechanism. This study explores two reaction mechanism hypotheses, involving one or two water molecules, and distinct roles for the Ca2+ cofactor. Our research focuses on the human synovial sPLA2 enzyme bound to lipid bilayers of varying phospholipid compositions, and employing adiabatic QM/MM and QM/MM MD umbrella sampling methods to energetically and geometrically characterize the structures found along both reaction pathways. Our studies demonstrate the higher frequency of productive conformations within the single-water pathway, also revealing a lower free energy barrier for hydrolyzing POPC. Furthermore, we observe that the TS of this concerted one-step reaction closely resembles transition state geometries observed in X-ray crystallography complexes featuring high-affinity transition state analogue inhibitors.
中文翻译:

用 QM/MM 方法重新审视磷脂酶 A2 催化的磷脂水解反应途径
分泌型磷脂酶A2(sPLA2)是Ca 2+ 依赖的、广泛分布于几乎所有哺乳动物组织和细菌中的酶超家族。它也是几乎所有蛇以及许多无脊椎动物物种毒液的重要组成部分。在无毒环境中,sPLA2 通过结合细胞膜并催化磷脂 sn-2 位点的酯键水解,在细胞信号传导通路中发挥重要作用。在关节炎患者的滑液中检测到 GIIA sPLA2 水平升高,它表现出促炎功能。因此,识别 sPLA2 抑制剂有望创造高效的药物治疗方法。除了关节炎之外,GIIA sPLA2 之间的相似性为开发针对蛇咬伤的治疗方法提供了机会,蛇咬伤是最致命的被忽视的热带疾病。尽管经过数十年的研究,PLA2 膜结合、底物结合和反应机制的细节仍然难以捉摸,需要全面了解 sPLA2 催化机制。本研究探讨了两种反应机制假设,涉及一个或两个水分子,以及 Ca 2+ 辅助因子的不同作用。我们的研究重点是与不同磷脂成分的脂质双层结合的人类滑膜 sPLA2 酶,并采用绝热 QM/MM 和 QM/MM MD 伞形采样方法来从能量和几何角度表征沿两条反应途径发现的结构。我们的研究表明,单水途径中生产性构象的频率更高,也揭示了水解 POPC 的自由能垒较低。 此外,我们观察到这种协同一步反应的 TS 与在具有高亲和力过渡态类似物抑制剂的 X 射线晶体学复合物中观察到的过渡态几何形状非常相似。
更新日期:2024-05-22
中文翻译:

用 QM/MM 方法重新审视磷脂酶 A2 催化的磷脂水解反应途径
分泌型磷脂酶A2(sPLA2)是Ca 2+ 依赖的、广泛分布于几乎所有哺乳动物组织和细菌中的酶超家族。它也是几乎所有蛇以及许多无脊椎动物物种毒液的重要组成部分。在无毒环境中,sPLA2 通过结合细胞膜并催化磷脂 sn-2 位点的酯键水解,在细胞信号传导通路中发挥重要作用。在关节炎患者的滑液中检测到 GIIA sPLA2 水平升高,它表现出促炎功能。因此,识别 sPLA2 抑制剂有望创造高效的药物治疗方法。除了关节炎之外,GIIA sPLA2 之间的相似性为开发针对蛇咬伤的治疗方法提供了机会,蛇咬伤是最致命的被忽视的热带疾病。尽管经过数十年的研究,PLA2 膜结合、底物结合和反应机制的细节仍然难以捉摸,需要全面了解 sPLA2 催化机制。本研究探讨了两种反应机制假设,涉及一个或两个水分子,以及 Ca 2+ 辅助因子的不同作用。我们的研究重点是与不同磷脂成分的脂质双层结合的人类滑膜 sPLA2 酶,并采用绝热 QM/MM 和 QM/MM MD 伞形采样方法来从能量和几何角度表征沿两条反应途径发现的结构。我们的研究表明,单水途径中生产性构象的频率更高,也揭示了水解 POPC 的自由能垒较低。 此外,我们观察到这种协同一步反应的 TS 与在具有高亲和力过渡态类似物抑制剂的 X 射线晶体学复合物中观察到的过渡态几何形状非常相似。

































 京公网安备 11010802027423号
京公网安备 11010802027423号