当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of hydroxylammonium chloride as a reductant for hydrochloric acid leaching of valuable metals from discarded lithium-ion batteries
Hydrometallurgy ( IF 4.7 ) Pub Date : 2024-04-11 , DOI: 10.1016/j.hydromet.2024.106305 Maria del Mar Cerrillo-Gonzalez , Maria Villen-Guzman , Brahim Arhoun , Cesar Gomez-Lahoz , Carlos Vereda-Alonso
Hydrometallurgy ( IF 4.7 ) Pub Date : 2024-04-11 , DOI: 10.1016/j.hydromet.2024.106305 Maria del Mar Cerrillo-Gonzalez , Maria Villen-Guzman , Brahim Arhoun , Cesar Gomez-Lahoz , Carlos Vereda-Alonso
|
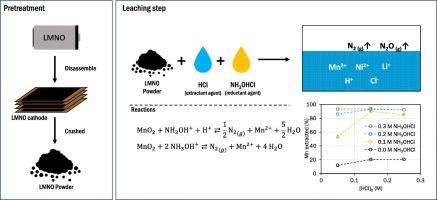
The use of hydroxylammonium chloride as a reducing agent is proposed to enhance the acid leaching of cathode material from spent lithium-ion batteries. The current study was conducted using real waste from scooter batteries. The main metals found in this cathode material are Mn (43.6%), Li (4.2%), Ni (8.3%) and Co (2.6%), expressed as % w/w. The effect of the initial concentrations of the extracting agent (HCl) and the reducing agent (NHOHCl) on the extraction yields of the target metals is investigated. The presence of NHOHCl in HCl solutions exerts a highly effective influence during the initial 15 min of the leaching process, with a complete solubilization of Mn within that timeframe, in contrast to the 20% achieved in its absence. During that period, over 70% of Li is solubilized, while Ni and Co reach maximum solubilities of 7% and 5%, respectively. Extending the contact time to 24 h between the extracting solution and LIB waste enables nearly complete extraction of Ni and exceeds 60% for Co. An analysis of variance was used to identify significant factors to be included in multivariable regressions to predict extraction yields. These regressions are used to carry out a preliminary economic analysis of the leaching process based on gross profit. The optimum outcome is achieved when the extraction is conducted through two consecutive leaching processes. In the first process, 100% Mn and 75% of Li are recovered, while the second process recovers the remaining Li, 96% of Ni, and 60% of cobalt. Additionally, the stoichiometry of the reduction of manganese(IV) by NHOHCl is studied through the correlation between the gas volume released during the leaching processes and the Mn solubilization reached. This reduction proceeds through two parallel reactions, resulting in the production of NO and N. The first of these reactions predominates, exhibiting an estimated selectivity of 87%.
中文翻译:

氯化羟铵作为还原剂对废旧锂离子电池盐酸浸出有价金属的影响
建议使用氯化羟铵作为还原剂来增强废旧锂离子电池正极材料的酸浸出。目前的研究是使用踏板车电池的真实废物进行的。该正极材料中的主要金属为锰 (43.6%)、锂 (4.2%)、镍 (8.3%) 和钴 (2.6%),以 % w/w 表示。研究了萃取剂 (HCl) 和还原剂 (NHOHCl) 的初始浓度对目标金属萃取率的影响。 HCl 溶液中 NHOHCl 的存在在浸出过程的最初 15 分钟内发挥了非常有效的影响,在该时间内锰完全溶解,而在不存在 NHOHCl 的情况下则达到了 20%。在此期间,超过 70% 的 Li 被溶解,而 Ni 和 Co 分别达到最大溶解度 7% 和 5%。将萃取溶液和 LIB 废物之间的接触时间延长至 24 小时,可以几乎完全萃取 Ni,并且 Co 的萃取率超过 60%。使用方差分析来确定要包含在多变量回归中的重要因素,以预测萃取率。这些回归用于根据毛利润对浸出过程进行初步经济分析。当通过两个连续的浸出过程进行提取时,可以获得最佳结果。在第一个过程中,回收了100%的Mn和75%的Li,而第二个过程则回收了剩余的Li、96%的Ni和60%的钴。此外,通过浸出过程中释放的气体体积与达到的锰溶解度之间的相关性,研究了 NHOHCl 还原锰 (IV) 的化学计量。 这种还原通过两个平行反应进行,产生 NO 和 N。其中第一个反应占主导地位,估计选择性为 87%。
更新日期:2024-04-11
中文翻译:

氯化羟铵作为还原剂对废旧锂离子电池盐酸浸出有价金属的影响
建议使用氯化羟铵作为还原剂来增强废旧锂离子电池正极材料的酸浸出。目前的研究是使用踏板车电池的真实废物进行的。该正极材料中的主要金属为锰 (43.6%)、锂 (4.2%)、镍 (8.3%) 和钴 (2.6%),以 % w/w 表示。研究了萃取剂 (HCl) 和还原剂 (NHOHCl) 的初始浓度对目标金属萃取率的影响。 HCl 溶液中 NHOHCl 的存在在浸出过程的最初 15 分钟内发挥了非常有效的影响,在该时间内锰完全溶解,而在不存在 NHOHCl 的情况下则达到了 20%。在此期间,超过 70% 的 Li 被溶解,而 Ni 和 Co 分别达到最大溶解度 7% 和 5%。将萃取溶液和 LIB 废物之间的接触时间延长至 24 小时,可以几乎完全萃取 Ni,并且 Co 的萃取率超过 60%。使用方差分析来确定要包含在多变量回归中的重要因素,以预测萃取率。这些回归用于根据毛利润对浸出过程进行初步经济分析。当通过两个连续的浸出过程进行提取时,可以获得最佳结果。在第一个过程中,回收了100%的Mn和75%的Li,而第二个过程则回收了剩余的Li、96%的Ni和60%的钴。此外,通过浸出过程中释放的气体体积与达到的锰溶解度之间的相关性,研究了 NHOHCl 还原锰 (IV) 的化学计量。 这种还原通过两个平行反应进行,产生 NO 和 N。其中第一个反应占主导地位,估计选择性为 87%。































 京公网安备 11010802027423号
京公网安备 11010802027423号