当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural characterization of E22G Aβ1–42 fibrils via 1H detected MAS NMR
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2024-04-26 , DOI: 10.1039/d4cp00553h Natalie Golota , Brian Michael , Edward P. Saliba , Sara Linse , Robert G. Griffin
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2024-04-26 , DOI: 10.1039/d4cp00553h Natalie Golota , Brian Michael , Edward P. Saliba , Sara Linse , Robert G. Griffin
|
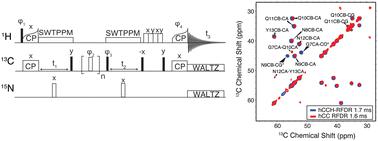
Amyloid fibrils have been implicated in the pathogenesis of several neurodegenerative diseases, the most prevalent example being Alzheimer's disease (AD). Despite the prevalence of AD, relatively little is known about the structure of the associated amyloid fibrils. This has motivated our studies of fibril structures, extended here to the familial Arctic mutant of Aβ1–42, E22G-Aβ1–42. We found E22G-AβM0,1–42 is toxic to Escherichia coli, thus we expressed E22G-Aβ1–42 fused to the self-cleavable tag NPro in the form of its EDDIE mutant. Since the high surface activity of E22G-Aβ1–42 makes it difficult to obtain more than sparse quantities of fibrils, we employed 1H detected magic angle spinning (MAS) nuclear magnetic resonance (NMR) experiments to characterize the protein. The 1H detected 13C–13C methods were first validated by application to fully protonated amyloidogenic nanocrystals of GNNQQNY, and then applied to fibrils of the Arctic mutant of Aβ, E22G-Aβ1–42. The MAS NMR spectra indicate that the biosynthetic samples of E22G-Aβ1–42 fibrils comprise a single conformation with 13C chemical shifts extracted from hCH, hNH, and hCCH spectra that are very similar to those of wild type Aβ1–42 fibrils. These results suggest that E22G-Aβ1–42 fibrils have a structure similar to that of wild type Aβ1–42.
中文翻译:

通过 1H 检测的 MAS NMR 表征 E22G Aβ1-42 原纤维的结构
淀粉样原纤维与多种神经退行性疾病的发病机制有关,最常见的例子是阿尔茨海默病(AD)。尽管 AD 很普遍,但人们对相关淀粉样原纤维的结构知之甚少。这激发了我们对原纤维结构的研究,在这里扩展到 Aβ 1-42、E22G-Aβ 1-42的家族性北极突变体。我们发现E22G-Aβ M0,1-42对大肠杆菌有毒,因此我们以EDDIE突变体的形式表达与自切割标签N Pro融合的E22G-Aβ 1-42 。由于 E22G-Aβ 1–42的高表面活性使其难以获得稀疏数量的原纤维,因此我们采用1 H 检测魔角旋转 (MAS) 核磁共振 (NMR) 实验来表征该蛋白质。1 H 检测13 C– 13 C方法首先通过应用于完全质子化的 GNNQQNY 淀粉样蛋白纳米晶体进行验证,然后应用于 Aβ 北极突变体 E22G-Aβ 1–42的原纤维。 MAS NMR 谱表明,E22G-Aβ 1-42原纤维的生物合成样品包含从 hCH、hNH 和 hCCH 谱中提取的具有13 C 化学位移的单一构象,与野生型 Aβ 1-42原纤维的构象非常相似。这些结果表明E22G-Aβ 1-42原纤维具有与野生型Aβ 1-42相似的结构。
更新日期:2024-04-26
中文翻译:

通过 1H 检测的 MAS NMR 表征 E22G Aβ1-42 原纤维的结构
淀粉样原纤维与多种神经退行性疾病的发病机制有关,最常见的例子是阿尔茨海默病(AD)。尽管 AD 很普遍,但人们对相关淀粉样原纤维的结构知之甚少。这激发了我们对原纤维结构的研究,在这里扩展到 Aβ 1-42、E22G-Aβ 1-42的家族性北极突变体。我们发现E22G-Aβ M0,1-42对大肠杆菌有毒,因此我们以EDDIE突变体的形式表达与自切割标签N Pro融合的E22G-Aβ 1-42 。由于 E22G-Aβ 1–42的高表面活性使其难以获得稀疏数量的原纤维,因此我们采用1 H 检测魔角旋转 (MAS) 核磁共振 (NMR) 实验来表征该蛋白质。1 H 检测13 C– 13 C方法首先通过应用于完全质子化的 GNNQQNY 淀粉样蛋白纳米晶体进行验证,然后应用于 Aβ 北极突变体 E22G-Aβ 1–42的原纤维。 MAS NMR 谱表明,E22G-Aβ 1-42原纤维的生物合成样品包含从 hCH、hNH 和 hCCH 谱中提取的具有13 C 化学位移的单一构象,与野生型 Aβ 1-42原纤维的构象非常相似。这些结果表明E22G-Aβ 1-42原纤维具有与野生型Aβ 1-42相似的结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号