当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Capillary Condensation of Water in Graphene Nanocapillaries
Nano Letters ( IF 10.8 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.nanolett.4c01088 Fahim Faraji 1, 2, 3 , Erik C. Neyts 1, 3 , Milorad V. Milošević 2, 3, 4 , François M. Peeters 2, 3, 5
Nano Letters ( IF 10.8 ) Pub Date : 2024-04-25 , DOI: 10.1021/acs.nanolett.4c01088 Fahim Faraji 1, 2, 3 , Erik C. Neyts 1, 3 , Milorad V. Milošević 2, 3, 4 , François M. Peeters 2, 3, 5
Affiliation
|
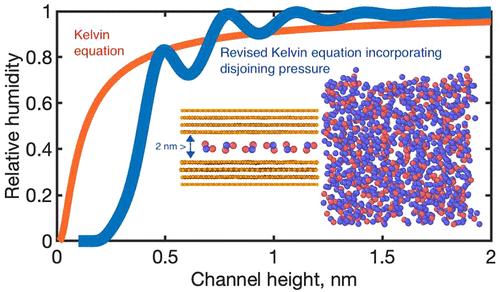
Recent experiments have revealed that the macroscopic Kelvin equation remains surprisingly accurate even for nanoscale capillaries. This phenomenon was so far explained by the oscillatory behavior of the solid–liquid interfacial free energy. We here demonstrate thermodynamic and capillarity inconsistencies with this explanation. After revising the Kelvin equation, we ascribe its validity at nanoscale confinement to the effect of disjoining pressure. To substantiate our hypothesis, we employed molecular dynamics simulations to evaluate interfacial heat transfer and wetting properties. Our assessments unveil a breakdown in a previously established proportionality between the work of adhesion and the Kapitza conductance at capillary heights below 1.3 nm, where the dominance of the work of adhesion shifts primarily from energy to entropy. Alternatively, the peak density of the initial water layer can effectively probe the work of adhesion. Unlike under bulk conditions, high confinement renders the work of adhesion entropically unfavorable.
中文翻译:

石墨烯纳米毛细管中水的毛细管冷凝
最近的实验表明,即使对于纳米级毛细管,宏观开尔文方程仍然令人惊讶地准确。迄今为止,这种现象可以通过固液界面自由能的振荡行为来解释。我们在这里证明了热力学和毛细管现象与这种解释的不一致。修改开尔文方程后,我们将其在纳米级约束下的有效性归因于分离压力的影响。为了证实我们的假设,我们采用分子动力学模拟来评估界面传热和润湿特性。我们的评估揭示了先前建立的在毛细管高度低于 1.3 nm 时粘附功和卡皮查电导之间的比例被打破,其中粘附功的主导地位主要从能量转移到熵。或者,初始水层的峰值密度可以有效地探测粘附功。与散装条件不同,高限制使得粘附功在熵上变得不利。
更新日期:2024-04-26
中文翻译:

石墨烯纳米毛细管中水的毛细管冷凝
最近的实验表明,即使对于纳米级毛细管,宏观开尔文方程仍然令人惊讶地准确。迄今为止,这种现象可以通过固液界面自由能的振荡行为来解释。我们在这里证明了热力学和毛细管现象与这种解释的不一致。修改开尔文方程后,我们将其在纳米级约束下的有效性归因于分离压力的影响。为了证实我们的假设,我们采用分子动力学模拟来评估界面传热和润湿特性。我们的评估揭示了先前建立的在毛细管高度低于 1.3 nm 时粘附功和卡皮查电导之间的比例被打破,其中粘附功的主导地位主要从能量转移到熵。或者,初始水层的峰值密度可以有效地探测粘附功。与散装条件不同,高限制使得粘附功在熵上变得不利。



























 京公网安备 11010802027423号
京公网安备 11010802027423号