当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Low Power Consumption Ammonia Electrosynthesis Using Hydrogen-Nitrate Flow Electrolyzer
ACS Energy Letters ( IF 22.0 ) Pub Date : 2024-04-22 , DOI: 10.1021/acsenergylett.4c00906 Qikun Hu 1 , Ouwen Peng 1 , Jia Liu 1 , Derong Chen 1 , Kian Ping Loh 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2024-04-22 , DOI: 10.1021/acsenergylett.4c00906 Qikun Hu 1 , Ouwen Peng 1 , Jia Liu 1 , Derong Chen 1 , Kian Ping Loh 1
Affiliation
|
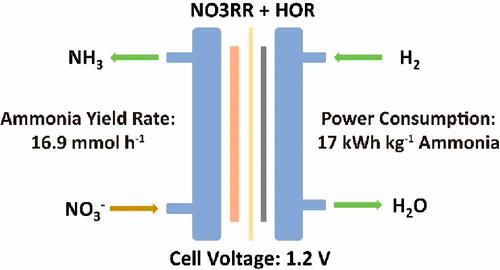
Ammonia synthesis through electrochemical nitrate reduction has emerged as a promising alternative to the conventional Haber–Bosch process. However, the use of a sluggish oxygen evolution reaction as the anode reaction leads to high energy consumption in nitrate reduction. In this study, we directly utilize hydrogen gas to synthesize ammonia by pairing the hydrogen oxidation reaction with the nitrate reduction reaction. A significantly lower cell voltage for ammonia synthesis was realized on a 16 cm2 flow electrolyzer. We achieved an impressive ammonia yield rate of 16.9 mmol h–1 at a cell voltage of 1.2 V cell voltage. Notably, this approach exhibits a low power consumption of 17 kWh kg–1 of NH3. The mechanism study shows hydroxyl ions generated from water splitting at the cathode cross the anion exchange membrane to react with protons generated from hydrogen oxidation at the anode. Through rigorous technical and economic analyses, this approach is found to be economically viable for industrial synthesis.
中文翻译:

低功耗硝酸氢流动电解槽电合成氨
通过电化学硝酸盐还原合成氨已成为传统哈伯-博世工艺的一种有前景的替代方案。然而,使用缓慢的析氧反应作为阳极反应会导致硝酸盐还原过程中的高能耗。在本研究中,我们通过将氢气氧化反应与硝酸盐还原反应配对,直接利用氢气合成氨。在16 cm 2流量电解槽上实现了氨合成的显着较低的电池电压。我们在 1.2 V 电池电压下实现了令人印象深刻的 16.9 mmol h –1氨产率。值得注意的是,该方法表现出 17 kWh kg –1 NH 3的低功耗。机理研究表明,阴极水分解产生的氢氧根离子穿过阴离子交换膜,与阳极氢氧化产生的质子发生反应。通过严格的技术和经济分析,发现这种方法对于工业合成来说在经济上是可行的。
更新日期:2024-04-26
中文翻译:

低功耗硝酸氢流动电解槽电合成氨
通过电化学硝酸盐还原合成氨已成为传统哈伯-博世工艺的一种有前景的替代方案。然而,使用缓慢的析氧反应作为阳极反应会导致硝酸盐还原过程中的高能耗。在本研究中,我们通过将氢气氧化反应与硝酸盐还原反应配对,直接利用氢气合成氨。在16 cm 2流量电解槽上实现了氨合成的显着较低的电池电压。我们在 1.2 V 电池电压下实现了令人印象深刻的 16.9 mmol h –1氨产率。值得注意的是,该方法表现出 17 kWh kg –1 NH 3的低功耗。机理研究表明,阴极水分解产生的氢氧根离子穿过阴离子交换膜,与阳极氢氧化产生的质子发生反应。通过严格的技术和经济分析,发现这种方法对于工业合成来说在经济上是可行的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号