当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Fully Methylated Cyclic Ether Solvent Enables Graphite Anode Cycling at Low Temperatures
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2024-04-24 , DOI: 10.1021/acsami.4c03149 Yongxin Ma 1 , Minghao Huang 1 , Yejuan Xue 1 , Xiaolong Wu 1 , Weilong Kong 1 , Yuxin Zhou 1 , Heng Zhang 1 , Hongfa Xiang 1 , Zhimei Huang 1
ACS Applied Materials & Interfaces ( IF 9.5 ) Pub Date : 2024-04-24 , DOI: 10.1021/acsami.4c03149 Yongxin Ma 1 , Minghao Huang 1 , Yejuan Xue 1 , Xiaolong Wu 1 , Weilong Kong 1 , Yuxin Zhou 1 , Heng Zhang 1 , Hongfa Xiang 1 , Zhimei Huang 1
Affiliation
|
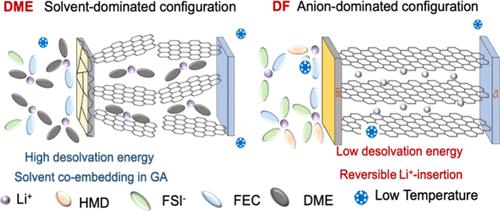
Graphite anode suffers from great capacity loss and larger cell polarization under low-temperature conditions in lithium-ion batteries (LIBs), which are mainly caused by the high energy barrier for the Li+ desolvation process and sluggish Li+ transfer rate across the solid electrolyte interface (SEI). Regulating an electrolyte with an anion-dominated solvation structure could synchronously stabilize the interface and boost the reaction kinetics of the graphite anode. Herein, a highly ionic conductive electrolyte consisting of a fully methylated cyclic ether solvent of 2,2,4,4,5,5-hexamethyl-1,3-dioxolane (HMD) and fluoroethylene carbonate (FEC) cosolvent was designed. The high electron-donating effect and steric hindrance of −(CH3)2 in HMD endow the HMD-based electrolyte with high ionic conductivity but lower coordination numbers with Li+, and an anion-dominated solvation structure was formed. Such configuration can accelerate the desolvation process and induce the forming of a LiF-rich SEI film on the anode, avoiding the solvent coembedding into graphite and enhancing the ion migration rate under low temperatures. The assembled Li||graphite cell with the tame electrolyte outperformed the conventional carbonates-based cell, showing 93.8% capacity retention after 227 cycles for the DF-based cell compared to 64.7% after 150 cycles. It also exhibited a prolonged cycle life for 200 rounds with 81% capacity retention under −20 °C. Therefore, this work offers a valuable thought for solvent design and provides approaches to electrolyte design for low-temperature LIBs.
中文翻译:

全甲基化环醚溶剂使石墨阳极能够在低温下循环
锂离子电池(LIB)中石墨负极在低温条件下会出现较大的容量损失和较大的电池极化,这主要是由于Li +去溶剂化过程的高能垒和Li +在固体电解质中的传输速率缓慢造成的接口(SEI)。用阴离子为主的溶剂化结构调节电解质可以同步稳定界面并提高石墨阳极的反应动力学。在此,设计了一种由完全甲基化的环醚溶剂2,2,4,4,5,5-六甲基-1,3-二氧戊环(HMD)和氟代碳酸亚乙酯(FEC)共溶剂组成的高离子导电电解质。 HMD中-(CH 3 ) 2的高给电子效应和位阻赋予HMD基电解质高离子电导率但与Li + 的配位数较低,并形成阴离子主导的溶剂化结构。这种配置可以加速去溶剂化过程并诱导在阳极上形成富含LiF的SEI膜,避免溶剂共嵌入石墨中并提高低温下的离子迁移速率。使用温和电解质组装而成的锂电池的性能优于传统碳酸盐电池,DF 基电池在 227 次循环后容量保持率为 93.8%,而 150 次循环后容量保持率为 64.7%。它还表现出 200 轮循环寿命的延长,在 -20 °C 下容量保持率为 81%。因此,这项工作为溶剂设计提供了宝贵的思路,并提供了低温锂离子电池电解质设计的方法。
更新日期:2024-04-26
中文翻译:

全甲基化环醚溶剂使石墨阳极能够在低温下循环
锂离子电池(LIB)中石墨负极在低温条件下会出现较大的容量损失和较大的电池极化,这主要是由于Li +去溶剂化过程的高能垒和Li +在固体电解质中的传输速率缓慢造成的接口(SEI)。用阴离子为主的溶剂化结构调节电解质可以同步稳定界面并提高石墨阳极的反应动力学。在此,设计了一种由完全甲基化的环醚溶剂2,2,4,4,5,5-六甲基-1,3-二氧戊环(HMD)和氟代碳酸亚乙酯(FEC)共溶剂组成的高离子导电电解质。 HMD中-(CH 3 ) 2的高给电子效应和位阻赋予HMD基电解质高离子电导率但与Li + 的配位数较低,并形成阴离子主导的溶剂化结构。这种配置可以加速去溶剂化过程并诱导在阳极上形成富含LiF的SEI膜,避免溶剂共嵌入石墨中并提高低温下的离子迁移速率。使用温和电解质组装而成的锂电池的性能优于传统碳酸盐电池,DF 基电池在 227 次循环后容量保持率为 93.8%,而 150 次循环后容量保持率为 64.7%。它还表现出 200 轮循环寿命的延长,在 -20 °C 下容量保持率为 81%。因此,这项工作为溶剂设计提供了宝贵的思路,并提供了低温锂离子电池电解质设计的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号