当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hybrid adipocyte-derived exosome nano platform for potent chemo-phototherapy in targeted hepatocellular carcinoma
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2024-04-24 , DOI: 10.1016/j.jconrel.2024.04.031 Xinying Liu , Jiaxin Zhang , Shunzhe Zheng , Meng Li , Wenqian Xu , Jianbin Shi , Ken-ichiro Kamei , Chutong Tian
Journal of Controlled Release ( IF 10.8 ) Pub Date : 2024-04-24 , DOI: 10.1016/j.jconrel.2024.04.031 Xinying Liu , Jiaxin Zhang , Shunzhe Zheng , Meng Li , Wenqian Xu , Jianbin Shi , Ken-ichiro Kamei , Chutong Tian
|
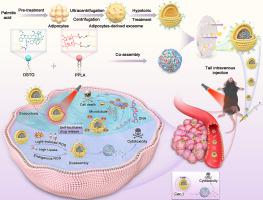
The high prevalence and severity of hepatocellular carcinoma (HCC) present a significant menace to human health. Despite the significant advancements in nanotechnology-driven antineoplastic agents, there remains a conspicuous gap in the development of targeted chemotherapeutic agents specifically designed for HCC. Consequently, there is an urgent need to explore potent drug delivery systems for effective HCC treatment. Here we have exploited the interplay between HCC and adipocyte to engineer a hybrid adipocyte-derived exosome platform, serving as a versatile vehicle to specifically target HCC and exsert potent antitumor effect. A lipid-like prodrug of docetaxel (DSTG) with a reactive oxygen species (ROS)-cleavable linker, and a lipid-conjugated photosensitizer (PPLA), spontaneously co-assemble into nanoparticles, functioning as the lipid cores of the hybrid exosomes (HEMPs and NEMPs). These nanoparticles are further encapsuled within adipocyte-derived exosome membranes, enhancing their affinity towards HCC cancer cells. As such, cancer cell uptakes of hybrid exosomes are increased up to 5.73-fold compared to lipid core nanoparticles. Our and experiments have demonstrated that HEMPs not only enhance the bioactivity of the prodrug and extend its circulation in the bloodstream but also effectively inhibit tumor growth by selectively targeting hepatocellular carcinoma tumor cells. Self-facilitated synergistic drug release subsequently promoting antitumor efficacy, inducing significant inhibition of tumor growth with minimal side effects. Our findings herald a promising direction for the development of targeted HCC therapeutics.
中文翻译:

混合脂肪细胞衍生的外泌体纳米平台,用于靶向肝细胞癌的有效化疗光疗
肝细胞癌(HCC)的高患病率和严重程度对人类健康构成重大威胁。尽管纳米技术驱动的抗肿瘤药物取得了重大进展,但在开发专为 HCC 设计的靶向化疗药物方面仍存在明显差距。因此,迫切需要探索有效的药物递送系统来有效治疗 HCC。在这里,我们利用 HCC 和脂肪细胞之间的相互作用来设计混合脂肪细胞衍生的外泌体平台,作为特异性靶向 HCC 并发挥有效抗肿瘤作用的多功能载体。多西紫杉醇 (DSTG) 的脂质样前药,具有活性氧 (ROS) 可裂解连接体和脂质缀合光敏剂 (PPLA),自发地共同组装成纳米颗粒,充当混合外泌体 (HEMP) 的脂质核心和 NEMP)。这些纳米颗粒进一步封装在脂肪细胞来源的外泌体膜内,增强了它们对 HCC 癌细胞的亲和力。因此,与脂质核心纳米颗粒相比,癌细胞对混合外泌体的摄取增加了 5.73 倍。我们的实验表明,HEMP不仅增强前药的生物活性并延长其在血流中的循环,而且还可以通过选择性靶向肝细胞癌肿瘤细胞来有效抑制肿瘤生长。自促进协同药物释放随后促进抗肿瘤功效,以最小的副作用诱导显着抑制肿瘤生长。我们的研究结果预示着肝癌靶向治疗药物开发的一个有希望的方向。
更新日期:2024-04-24
中文翻译:

混合脂肪细胞衍生的外泌体纳米平台,用于靶向肝细胞癌的有效化疗光疗
肝细胞癌(HCC)的高患病率和严重程度对人类健康构成重大威胁。尽管纳米技术驱动的抗肿瘤药物取得了重大进展,但在开发专为 HCC 设计的靶向化疗药物方面仍存在明显差距。因此,迫切需要探索有效的药物递送系统来有效治疗 HCC。在这里,我们利用 HCC 和脂肪细胞之间的相互作用来设计混合脂肪细胞衍生的外泌体平台,作为特异性靶向 HCC 并发挥有效抗肿瘤作用的多功能载体。多西紫杉醇 (DSTG) 的脂质样前药,具有活性氧 (ROS) 可裂解连接体和脂质缀合光敏剂 (PPLA),自发地共同组装成纳米颗粒,充当混合外泌体 (HEMP) 的脂质核心和 NEMP)。这些纳米颗粒进一步封装在脂肪细胞来源的外泌体膜内,增强了它们对 HCC 癌细胞的亲和力。因此,与脂质核心纳米颗粒相比,癌细胞对混合外泌体的摄取增加了 5.73 倍。我们的实验表明,HEMP不仅增强前药的生物活性并延长其在血流中的循环,而且还可以通过选择性靶向肝细胞癌肿瘤细胞来有效抑制肿瘤生长。自促进协同药物释放随后促进抗肿瘤功效,以最小的副作用诱导显着抑制肿瘤生长。我们的研究结果预示着肝癌靶向治疗药物开发的一个有希望的方向。



























 京公网安备 11010802027423号
京公网安备 11010802027423号