当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A ferroptosis amplifier based on triple-enhanced lipid peroxides accumulation strategy for effective pancreatic cancer therapy
Biomaterials ( IF 14.0 ) Pub Date : 2024-04-21 , DOI: 10.1016/j.biomaterials.2024.122574 Mengyao Chen , Xiaohan Tong , Yanting Sun , Chunyan Dong , Chen Li , Chunhui Wang , Minyi Zhang , Yixuan Wen , Pinting Ye , Ruihao Li , Jie Wan , Shujing Liang , Shuo Shi
Biomaterials ( IF 14.0 ) Pub Date : 2024-04-21 , DOI: 10.1016/j.biomaterials.2024.122574 Mengyao Chen , Xiaohan Tong , Yanting Sun , Chunyan Dong , Chen Li , Chunhui Wang , Minyi Zhang , Yixuan Wen , Pinting Ye , Ruihao Li , Jie Wan , Shujing Liang , Shuo Shi
|
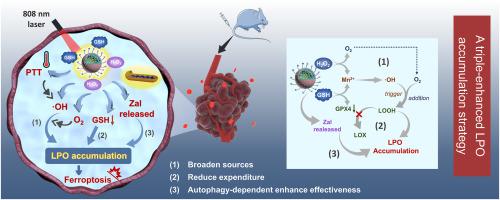
As an iron dependent regulatory cell death process driven by excessive lipid peroxides (LPO), ferroptosis is recognized as a powerful weapon for pancreatic cancer (PC) therapy. However, the tumor microenvironment (TME) with hypoxia and elevated glutathione (GSH) expression not only inhibits LPO production, but also induces glutathione peroxidase 4 (GPX4) mediated LPO clearance, which greatly compromise the therapeutic outcomes of ferroptosis. To address these issues, herein, a novel triple-enhanced ferroptosis amplifier (denoted as Zal@HM-PTBC) is rationally designed. After intravenous injection, the overexpressed HO/GSH in TME induces the collapse of Zal@HM-PTBC and triggers the production of oxygen and reactive oxygen species (ROS), which synergistically amplify the degree of lipid peroxidation (broaden sources). Concurrently, GSH consumption because of the degradation of the hollow manganese dioxide (HM) significantly weakens the activity of GPX4, resulting in a decrease in LPO clearance (reduce expenditure). Moreover, the loading and site-directed release of zalcitabine further promotes autophagy-dependent LPO accumulation (enhance effectiveness). Both and results validated that the ferroptosis amplifier demonstrated superior specificity and favorable therapeutic responses. Overall, this triple-enhanced LPO accumulation strategy demonstrates the ability to facilitate the efficacy of ferroptosis, injecting vigorous vitality into the treatment of PC.
中文翻译:

基于三重增强脂质过氧化物积累策略的铁死亡放大器,用于有效治疗胰腺癌
作为由过量脂质过氧化物(LPO)驱动的铁依赖性调节性细胞死亡过程,铁死亡被认为是胰腺癌(PC)治疗的有力武器。然而,缺氧和谷胱甘肽(GSH)表达升高的肿瘤微环境(TME)不仅抑制LPO产生,还诱导谷胱甘肽过氧化物酶4(GPX4)介导的LPO清除,这极大地影响了铁死亡的治疗效果。为了解决这些问题,本文合理设计了一种新型三重增强铁死亡放大器(表示为Zal@HM-PTBC)。静脉注射后,TME 中过表达的 HO/GSH 会诱导 Zal@HM-PTBC 崩溃,并引发氧气和活性氧 (ROS) 的产生,从而协同放大脂质过氧化程度(拓宽来源)。同时,由于空心二氧化锰(HM)的降解而消耗的GSH显着削弱了GPX4的活性,导致LPO清除率下降(减少支出)。此外,扎西他滨的负载和定点释放进一步促进自噬依赖性LPO积累(增强有效性)。结果均证实铁死亡放大器表现出卓越的特异性和良好的治疗反应。总体而言,这种三重增强的LPO积累策略展示了促进铁死亡功效的能力,为PC的治疗注入了旺盛的活力。
更新日期:2024-04-21
中文翻译:

基于三重增强脂质过氧化物积累策略的铁死亡放大器,用于有效治疗胰腺癌
作为由过量脂质过氧化物(LPO)驱动的铁依赖性调节性细胞死亡过程,铁死亡被认为是胰腺癌(PC)治疗的有力武器。然而,缺氧和谷胱甘肽(GSH)表达升高的肿瘤微环境(TME)不仅抑制LPO产生,还诱导谷胱甘肽过氧化物酶4(GPX4)介导的LPO清除,这极大地影响了铁死亡的治疗效果。为了解决这些问题,本文合理设计了一种新型三重增强铁死亡放大器(表示为Zal@HM-PTBC)。静脉注射后,TME 中过表达的 HO/GSH 会诱导 Zal@HM-PTBC 崩溃,并引发氧气和活性氧 (ROS) 的产生,从而协同放大脂质过氧化程度(拓宽来源)。同时,由于空心二氧化锰(HM)的降解而消耗的GSH显着削弱了GPX4的活性,导致LPO清除率下降(减少支出)。此外,扎西他滨的负载和定点释放进一步促进自噬依赖性LPO积累(增强有效性)。结果均证实铁死亡放大器表现出卓越的特异性和良好的治疗反应。总体而言,这种三重增强的LPO积累策略展示了促进铁死亡功效的能力,为PC的治疗注入了旺盛的活力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号