当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electric Potential-Driven Acid/Base Chemistry: Kinetics of Electrochemical Interfacial Proton Transfer and Transport
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2024-04-22 , DOI: 10.1021/acs.jpcc.4c01114 Andrew D. Pendergast 1 , Katherine J. Levey 2, 3 , Julie V. Macpherson 2, 3 , Martin A. Edwards 4 , Henry S. White 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2024-04-22 , DOI: 10.1021/acs.jpcc.4c01114 Andrew D. Pendergast 1 , Katherine J. Levey 2, 3 , Julie V. Macpherson 2, 3 , Martin A. Edwards 4 , Henry S. White 1
Affiliation
|
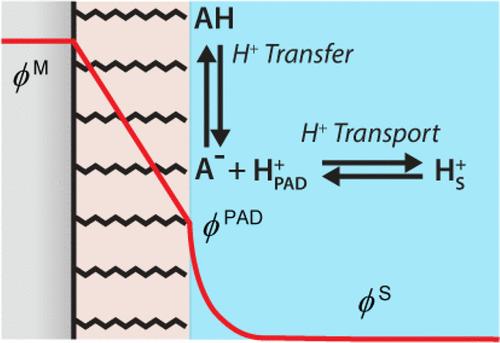
Proton transfer at solid/liquid interfaces is a fundamental step in many complex biological and electrocatalytic processes. Previous model studies using electrodes modified with self-assembled monolayers (SAMs) of carboxylic acid-terminated alkanethiols have demonstrated that interfacial proton transfer is controlled by the local electrochemical microenvironment. The thermodynamic driving force for electrochemically driven protonation/deprotonation of acid/base SAMs is governed by a combination of the electric potential at the SAM/solvent interface, the pKa of the acid group, and the solution pH. Here, we develop a kinetic model to describe electric potential-driven protonation/deprotonation as a two-step process. This comprises a reversible proton transfer step at the SAM/electrolyte interface (i.e., (de)protonation) and a proton transport step describing the motion of protons as they traverse the diffuse electrical double layer to and from the solution bulk. The kinetics of the transport step are investigated using finite element simulations, providing numerical estimates for the transport rate constants under combined diffusional and migrational transport modes. Using the dependence of these rate constants on the electric potential at the SAM/electrolyte interface, we define situations where the overall rate expression is limited by either (de)protonation, proton transport, or a combination of both. From this analysis, we determine a lower limit for the acid group pKa of ≈3, above which proton transfer at the plane of acid dissociation is generally the rate-determining step. The electric potential-driven proton transfer/transport kinetic model developed herein provides a general approach to treat electric potential-driven coupled ion transfer and transport phenomena, with potential applications including proton-coupled electron transfer processes, ion intercalation in alkali metal batteries, and ion transport across biological membranes.
中文翻译:

电势驱动的酸/碱化学:电化学界面质子转移和传输的动力学
固/液界面的质子转移是许多复杂的生物和电催化过程的基本步骤。先前使用末端羧酸链烷硫醇自组装单层 (SAM) 修饰的电极进行的模型研究表明,界面质子转移受局部电化学微环境控制。电化学驱动的酸/碱 SAM 质子化/去质子化的热力学驱动力由 SAM/溶剂界面处的电势、酸基的 p Ka和溶液 pH 值共同控制。在这里,我们开发了一个动力学模型,将电势驱动的质子化/去质子化描述为两步过程。这包括 SAM/电解质界面处的可逆质子转移步骤(即(去)质子化)和描述质子在穿过扩散双电层进出溶液本体时的运动的质子传输步骤。使用有限元模拟研究传输步骤的动力学,提供组合扩散和迁移传输模式下的传输速率常数的数值估计。利用这些速率常数对 SAM/电解质界面电势的依赖性,我们定义了总体速率表达受(去)质子化、质子传输或两者的组合限制的情况。根据该分析,我们确定酸基 p Ka的下限为≈3,高于该下限,酸解离平面上的质子转移通常是速率决定步骤。本文开发的电势驱动的质子转移/传输动力学模型提供了处理电势驱动耦合离子转移和传输现象的通用方法,其潜在应用包括质子耦合电子转移过程、碱金属电池中的离子嵌入和离子跨生物膜运输。
更新日期:2024-04-22
中文翻译:

电势驱动的酸/碱化学:电化学界面质子转移和传输的动力学
固/液界面的质子转移是许多复杂的生物和电催化过程的基本步骤。先前使用末端羧酸链烷硫醇自组装单层 (SAM) 修饰的电极进行的模型研究表明,界面质子转移受局部电化学微环境控制。电化学驱动的酸/碱 SAM 质子化/去质子化的热力学驱动力由 SAM/溶剂界面处的电势、酸基的 p Ka和溶液 pH 值共同控制。在这里,我们开发了一个动力学模型,将电势驱动的质子化/去质子化描述为两步过程。这包括 SAM/电解质界面处的可逆质子转移步骤(即(去)质子化)和描述质子在穿过扩散双电层进出溶液本体时的运动的质子传输步骤。使用有限元模拟研究传输步骤的动力学,提供组合扩散和迁移传输模式下的传输速率常数的数值估计。利用这些速率常数对 SAM/电解质界面电势的依赖性,我们定义了总体速率表达受(去)质子化、质子传输或两者的组合限制的情况。根据该分析,我们确定酸基 p Ka的下限为≈3,高于该下限,酸解离平面上的质子转移通常是速率决定步骤。本文开发的电势驱动的质子转移/传输动力学模型提供了处理电势驱动耦合离子转移和传输现象的通用方法,其潜在应用包括质子耦合电子转移过程、碱金属电池中的离子嵌入和离子跨生物膜运输。



























 京公网安备 11010802027423号
京公网安备 11010802027423号