当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
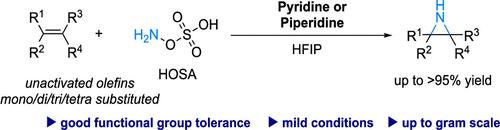
Synthesis of N–H Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids as Aminating Agent
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-23 , DOI: 10.1021/acs.joc.4c00253 Yi Huang 1 , Shi-Yang Zhu 1 , Gang He 1 , Gong Chen 1 , Hao Wang 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2024-04-23 , DOI: 10.1021/acs.joc.4c00253 Yi Huang 1 , Shi-Yang Zhu 1 , Gang He 1 , Gong Chen 1 , Hao Wang 1
Affiliation
|
Herein, we presented a practical methodology for the intermolecular aziridination of alkenes, using HOSA as the aminating agent, alongside pyridine or piperidine as the base, within HFIP solvent system. Notably, this approach showcases excellent reactivity, especially with nonactivated alkenes, and facilitates the transformation of various alkenes substrates, including mono-, di-, tri, and tetra-substituted alkenes, into aziridines with moderate to excellent yield. This method presents a promising avenue for synthesizing aziridines from a wide range of alkenes, featuring the benefits of straightforward operation, mild reaction conditions, extensive substrate compatibility, and scalability.
中文翻译:

以羟胺邻磺酸为胺化剂从未活化烯烃合成 N-H 氮丙啶
在此,我们提出了一种在 HFIP 溶剂体系中使用 HOSA 作为胺化剂,并以吡啶或哌啶作为碱进行烯烃分子间氮丙啶化的实用方法。值得注意的是,该方法表现出优异的反应性,特别是对于未活化的烯烃,并促进各种烯烃底物(包括单、二、三和四取代的烯烃)转化为氮丙啶,产率中等至优异。该方法为从多种烯烃合成氮丙啶提供了一种有前途的途径,具有操作简单、反应条件温和、底物广泛兼容性和可扩展性等优点。
更新日期:2024-04-23
中文翻译:

以羟胺邻磺酸为胺化剂从未活化烯烃合成 N-H 氮丙啶
在此,我们提出了一种在 HFIP 溶剂体系中使用 HOSA 作为胺化剂,并以吡啶或哌啶作为碱进行烯烃分子间氮丙啶化的实用方法。值得注意的是,该方法表现出优异的反应性,特别是对于未活化的烯烃,并促进各种烯烃底物(包括单、二、三和四取代的烯烃)转化为氮丙啶,产率中等至优异。该方法为从多种烯烃合成氮丙啶提供了一种有前途的途径,具有操作简单、反应条件温和、底物广泛兼容性和可扩展性等优点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号