当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Preparation of the Tricyclic Hexasubstituted Spirocyclopropane Core of Cyclohelminthol X
Organic Letters ( IF 5.2 ) Pub Date : 2024-04-24 , DOI: 10.1021/acs.orglett.4c00203 Kazuki Hashimoto 1 , Hayato Maeda 1 , Masaru Hashimoto 1
Organic Letters ( IF 5.2 ) Pub Date : 2024-04-24 , DOI: 10.1021/acs.orglett.4c00203 Kazuki Hashimoto 1 , Hayato Maeda 1 , Masaru Hashimoto 1
Affiliation
|
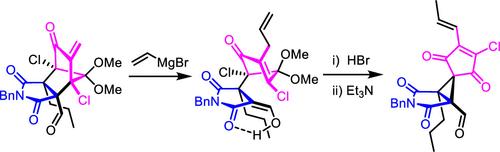
This study focused on synthesizing the tricyclic hexasubstituted spirocyclopropane-core framework 2 of cyclohelminthol X (1), an antifungal cytotoxin isolated from Helminthosporium velutinum yone96 in a stereoselective manner. The synthesis features an SN2-type cyclopropanation of the quaternary chloride 23 generated via a retro-Michael-type ring-opening reaction of an 8-azatricyclo[4.3.0.12,5]deca-3,7,9-trione derivative 22. The successful synthesis confirmed the structure of 1, resolving the ambiguity from the absence of X-ray crystallographic analysis. The prepared models exhibited potent cytotoxicity.
中文翻译:

环蠕虫醇X的三环六取代螺环丙烷核的立体选择性制备
本研究的重点是合成环蠕虫醇 X ( 1 ) 的三环六取代螺环丙烷核心框架2 ,环蠕虫醇 X 是一种以立体选择性方式从Helminthosporium velutinum yone96中分离出来的抗真菌细胞毒素。该合成的特点是通过 8-氮杂三环[4.3.0.1 2,5 ]deca-3,7,9-trione 衍生物的逆迈克尔型开环反应生成季氯化物23的S N 2 型环丙烷化反应22 .成功的合成证实了1的结构,解决了由于缺乏X射线晶体学分析而产生的歧义。制备的模型表现出有效的细胞毒性。
更新日期:2024-04-25
中文翻译:

环蠕虫醇X的三环六取代螺环丙烷核的立体选择性制备
本研究的重点是合成环蠕虫醇 X ( 1 ) 的三环六取代螺环丙烷核心框架2 ,环蠕虫醇 X 是一种以立体选择性方式从Helminthosporium velutinum yone96中分离出来的抗真菌细胞毒素。该合成的特点是通过 8-氮杂三环[4.3.0.1 2,5 ]deca-3,7,9-trione 衍生物的逆迈克尔型开环反应生成季氯化物23的S N 2 型环丙烷化反应22 .成功的合成证实了1的结构,解决了由于缺乏X射线晶体学分析而产生的歧义。制备的模型表现出有效的细胞毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号