当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
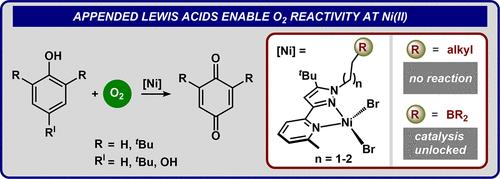
Appended Lewis Acids Enable Dioxygen Reactivity and Catalytic Oxidations with Ni(II)
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-25 , DOI: 10.1021/jacs.3c12399 Daniel M. Beagan 1 , Carolina Rivera 1 , Nathaniel K. Szymczak 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-25 , DOI: 10.1021/jacs.3c12399 Daniel M. Beagan 1 , Carolina Rivera 1 , Nathaniel K. Szymczak 1
Affiliation
|
We disclose a suite of Ni(II) complexes featuring secondary sphere Lewis acids of varied Lewis acidity and tether lengths. Several of these complexes feature atypical behavior of Ni(II): reactivity with O2 that occurs only in the presence of a tethered Lewis acid. In situ UV–vis spectroscopy revealed that, although adducts are stable at −40 °C, complexes containing 9-borabicyclo[3.3.1]nonane (9-BBN) Lewis acids underwent irreversible oxidative deborylation when warmed to room temperature. We computationally and experimentally identified that oxidative instability of appended 9-BBN moieties can be mitigated using weaker Lewis acids such as pinacolborane (BPin). These insights enabled the realization of catalytic reactions: hydrogen atom abstraction from phenols and room temperature oxygen atom transfer to PPh3.
中文翻译:

附加的路易斯酸可实现双氧反应并与 Ni(II) 进行催化氧化
我们公开了一套Ni(II)络合物,其特征在于具有不同路易斯酸度和链长度的次级球路易斯酸。其中一些配合物具有 Ni(II) 的非典型行为:仅在存在束缚路易斯酸的情况下才会发生与 O 2 的反应。原位紫外可见光谱显示,虽然加合物在-40°C下稳定,但含有9-硼双环[3.3.1]壬烷(9-BBN)路易斯酸的络合物在升温至室温时会发生不可逆的氧化脱硼化。我们通过计算和实验发现,附加的 9-BBN 部分的氧化不稳定性可以使用较弱的路易斯酸(例如频哪醇硼烷 (BPin))来减轻。这些见解使得催化反应得以实现:从苯酚中提取氢原子以及室温下氧原子转移至 PPh 3。
更新日期:2024-04-25
中文翻译:

附加的路易斯酸可实现双氧反应并与 Ni(II) 进行催化氧化
我们公开了一套Ni(II)络合物,其特征在于具有不同路易斯酸度和链长度的次级球路易斯酸。其中一些配合物具有 Ni(II) 的非典型行为:仅在存在束缚路易斯酸的情况下才会发生与 O 2 的反应。原位紫外可见光谱显示,虽然加合物在-40°C下稳定,但含有9-硼双环[3.3.1]壬烷(9-BBN)路易斯酸的络合物在升温至室温时会发生不可逆的氧化脱硼化。我们通过计算和实验发现,附加的 9-BBN 部分的氧化不稳定性可以使用较弱的路易斯酸(例如频哪醇硼烷 (BPin))来减轻。这些见解使得催化反应得以实现:从苯酚中提取氢原子以及室温下氧原子转移至 PPh 3。



























 京公网安备 11010802027423号
京公网安备 11010802027423号