当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Probing the Structure Change and Interaction of Interfacial Water and Hydroxyl Intermediates on Ni(OH)2 Surface over Water Splitting
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-24 , DOI: 10.1021/jacs.4c00948 Huajie Ze 1 , Zhi-Lan Yang 1 , Mu-Lin Li 1 , Xia-Guang Zhang 2 , Yao-Lin A 1 , Qing-Na Zheng 1 , Yao-Hui Wang 1 , Jing-Hua Tian 3 , Yue-Jiao Zhang 1 , Jian-Feng Li 1, 3, 4
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-24 , DOI: 10.1021/jacs.4c00948 Huajie Ze 1 , Zhi-Lan Yang 1 , Mu-Lin Li 1 , Xia-Guang Zhang 2 , Yao-Lin A 1 , Qing-Na Zheng 1 , Yao-Hui Wang 1 , Jing-Hua Tian 3 , Yue-Jiao Zhang 1 , Jian-Feng Li 1, 3, 4
Affiliation
|
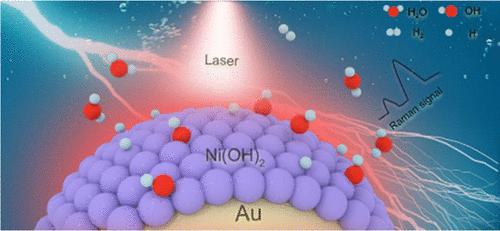
There is growing acknowledgment that the properties of the electrochemical interfaces play an increasingly pivotal role in improving the performance of the hydrogen evolution reaction (HER). Here, we present, for the first time, direct dynamic spectral evidence illustrating the impact of the interaction between interfacial water molecules and adsorbed hydroxyl species (OHad) on the HER properties of Ni(OH)2 using Au/core-Ni(OH)2/shell nanoparticle-enhanced Raman spectroscopy. Notably, our findings highlight that the interaction between OHad and interfacial water molecules promotes the formation of weakly hydrogen-bonded water, fostering an environment conducive to improving the HER performance. Furthermore, the participation of OHad in the reaction is substantiated by the observed deprotonation step of Au@2 nm Ni(OH)2 during the HER process. This phenomenon is corroborated by the phase transition of Ni(OH)2 to NiO, as verified through Raman and X-ray photoelectron spectroscopy. The significant redshift in the OH-stretching frequency of water molecules during the phase transition confirms that surface OHad disrupts the hydrogen-bond network of interfacial water molecules. Through manipulation of the shell thickness of Au@Ni(OH)2, we additionally validate the interaction between OHad and interfacial water molecules. In summary, our insights emphasize the potential of electrochemical interfacial engineering as a potent approach to enhance electrocatalytic performance.
中文翻译:

原位探测 Ni(OH)2 表面水分解过程中界面水与羟基中间体的结构变化和相互作用
人们越来越认识到电化学界面的特性在提高析氢反应(HER)性能方面发挥着越来越关键的作用。在这里,我们首次提出直接动态光谱证据,说明界面水分子和吸附羟基物种 (OH ad ) 之间的相互作用对使用 Au/core-Ni(OH ) 的 Ni(OH) 2的 HER 性质的影响) 2 /壳纳米颗粒增强拉曼光谱。值得注意的是,我们的研究结果强调,OH ad和界面水分子之间的相互作用促进弱氢键水的形成,营造有利于提高 HER 性能的环境。此外,在HER过程中观察到的Au@2 nm Ni(OH) 2的去质子化步骤证实了OH ad参与反应。 Ni(OH) 2到 NiO的相变证实了这一现象,并通过拉曼和 X 射线光电子能谱进行了验证。相变过程中水分子的 OH 伸缩频率的显着红移证实了表面 OH ad破坏了界面水分子的氢键网络。通过控制 Au@Ni(OH) 2的壳厚度,我们还验证了 OH ad与界面水分子之间的相互作用。总之,我们的见解强调了电化学界面工程作为增强电催化性能的有效方法的潜力。
更新日期:2024-04-25
中文翻译:

原位探测 Ni(OH)2 表面水分解过程中界面水与羟基中间体的结构变化和相互作用
人们越来越认识到电化学界面的特性在提高析氢反应(HER)性能方面发挥着越来越关键的作用。在这里,我们首次提出直接动态光谱证据,说明界面水分子和吸附羟基物种 (OH ad ) 之间的相互作用对使用 Au/core-Ni(OH ) 的 Ni(OH) 2的 HER 性质的影响) 2 /壳纳米颗粒增强拉曼光谱。值得注意的是,我们的研究结果强调,OH ad和界面水分子之间的相互作用促进弱氢键水的形成,营造有利于提高 HER 性能的环境。此外,在HER过程中观察到的Au@2 nm Ni(OH) 2的去质子化步骤证实了OH ad参与反应。 Ni(OH) 2到 NiO的相变证实了这一现象,并通过拉曼和 X 射线光电子能谱进行了验证。相变过程中水分子的 OH 伸缩频率的显着红移证实了表面 OH ad破坏了界面水分子的氢键网络。通过控制 Au@Ni(OH) 2的壳厚度,我们还验证了 OH ad与界面水分子之间的相互作用。总之,我们的见解强调了电化学界面工程作为增强电催化性能的有效方法的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号