当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Electrocatalytic Hydrogen Evolution by an Exceptionally Stable Cobalt Complex
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2024-04-19 , DOI: 10.1021/acs.inorgchem.4c01043 Maria B. Brands 1 , Joost N. H. Reek 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2024-04-19 , DOI: 10.1021/acs.inorgchem.4c01043 Maria B. Brands 1 , Joost N. H. Reek 1
Affiliation
|
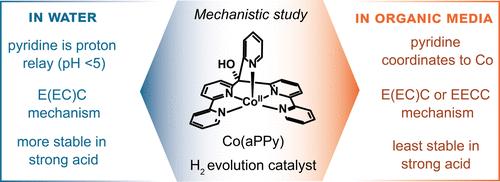
Co(aPPy) is one of the most stable and active molecular first-row transition-metal catalysts for proton reduction reported to date. Understanding the origin of its high performance via mechanistic studies could aid in developing even better catalysts. In this work, the catalytic mechanism of Co(aPPy) was electrochemically probed, in both organic solvents and water. We found that different mechanisms can occur depending on the solvent and the acidity of the medium. In organic solvent with a strong acid as the proton source, catalysis initiates directly after a single-electron reduction of CoII to CoI, whereas in the presence of a weaker acid, the cobalt center needs to be reduced twice before catalysis occurs. In the aqueous phase, we found drastically different electrochemical behavior, where the Co(aPPy) complex was found to be a precatalyst to a different electrocatalytic species. We propose that in this active catalyst, the pyridine ring has dissociated and acts as a proton relay at pH ≤ 5, which opens up a fast protonation pathway of the CoI intermediate and results in a high catalytic activity. Furthermore, we determined with constant potential bulk electrolysis that the catalyst is most stable at pH 3. The catalyst thus functions optimally at low pH in an aqueous environment, where the pyridine acts as a proton shuttle and where the high acidity also prevents catalyst deactivation.
中文翻译:

异常稳定的钴配合物电催化析氢的机理见解
Co(aPPy)是迄今为止报道的用于质子还原的最稳定和活性的分子第一行过渡金属催化剂之一。通过机理研究了解其高性能的起源可能有助于开发更好的催化剂。在这项工作中,我们在有机溶剂和水中对 Co(aPPy) 的催化机理进行了电化学探讨。我们发现,根据溶剂和介质的酸度,可能会发生不同的机制。在以强酸作为质子源的有机溶剂中,催化作用在Co II单电子还原为Co I后立即开始,而在弱酸存在下,钴中心需要还原两次才能发生催化作用。在水相中,我们发现了截然不同的电化学行为,其中 Co(aPPy) 络合物被发现是不同电催化物质的预催化剂。我们认为,在这种活性催化剂中,吡啶环在pH ≤ 5时解离并充当质子中继,从而打开了Co I中间体的快速质子化途径并产生高催化活性。此外,我们通过恒电位本体电解确定该催化剂在 pH 3 时最稳定。因此,该催化剂在水环境中的低 pH 条件下发挥最佳作用,其中吡啶充当质子梭,高酸度还可以防止催化剂失活。
更新日期:2024-04-19
中文翻译:

异常稳定的钴配合物电催化析氢的机理见解
Co(aPPy)是迄今为止报道的用于质子还原的最稳定和活性的分子第一行过渡金属催化剂之一。通过机理研究了解其高性能的起源可能有助于开发更好的催化剂。在这项工作中,我们在有机溶剂和水中对 Co(aPPy) 的催化机理进行了电化学探讨。我们发现,根据溶剂和介质的酸度,可能会发生不同的机制。在以强酸作为质子源的有机溶剂中,催化作用在Co II单电子还原为Co I后立即开始,而在弱酸存在下,钴中心需要还原两次才能发生催化作用。在水相中,我们发现了截然不同的电化学行为,其中 Co(aPPy) 络合物被发现是不同电催化物质的预催化剂。我们认为,在这种活性催化剂中,吡啶环在pH ≤ 5时解离并充当质子中继,从而打开了Co I中间体的快速质子化途径并产生高催化活性。此外,我们通过恒电位本体电解确定该催化剂在 pH 3 时最稳定。因此,该催化剂在水环境中的低 pH 条件下发挥最佳作用,其中吡啶充当质子梭,高酸度还可以防止催化剂失活。



























 京公网安备 11010802027423号
京公网安备 11010802027423号