Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water-polymer interactions and mechanisms of water-driven glass transition decrease in non-isocyanate polyhydroxyurethanes with varying hydration sites
Polymer ( IF 4.6 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.polymer.2024.127060 Izabela Łukaszewska , Artur Bukowczan , Konstantinos N. Raftopoulos , Krzysztof Pielichowski
Polymer ( IF 4.6 ) Pub Date : 2024-04-16 , DOI: 10.1016/j.polymer.2024.127060 Izabela Łukaszewska , Artur Bukowczan , Konstantinos N. Raftopoulos , Krzysztof Pielichowski
|
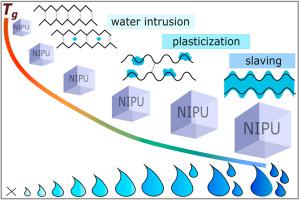
Non-isocyanate polyurethanes (NIPUs) with varying content of secondary amino groups along their chain were studied with respect to water absorption and plasticization. Secondary amino groups promote water uptake. Water sorption isotherms in the water activity range 0–0.97 are discussed in terms of various sorption models. Secondary amino groups act as additional hydration sites, increasing monolayer capacity. The red shift of the IR band assigned to the carbonyl of the urethane and the simultaneous blue shift of the urethane N–H bending band show cleavage of the polymer-polymer hydrogen bonds upon water uptake, due to strong interactions between water molecules and hydroxyurethanes constituting the primary hydration sites. A decrease in glass transition temperature () is observed with increasing water content. The effect is discussed in terms of plasticization and slaving mechanisms, while a peculiar decrease of at very low hydrations is attributed to a mechanism not following common mixing laws.
中文翻译:

具有不同水合位点的非异氰酸酯多羟基聚氨酯中水-聚合物相互作用和水驱动玻璃化转变降低的机制
研究了沿链具有不同仲氨基含量的非异氰酸酯聚氨酯(NIPU)的吸水性和增塑性。仲氨基促进水的吸收。根据各种吸附模型讨论了水活度范围 0-0.97 内的水吸附等温线。仲氨基充当额外的水合位点,增加单层容量。分配给氨基甲酸乙酯羰基的红外谱带红移,同时氨基甲酸乙酯 N-H 弯曲谱带蓝移,表明由于水分子和羟基氨基甲酸酯之间的强烈相互作用,聚合物-聚合物氢键在吸水时断裂。主要水合位点。随着水含量的增加,观察到玻璃化转变温度 () 降低。该效应是根据塑化和奴役机制进行讨论的,而在非常低的水合作用下的特殊下降归因于不遵循常见混合定律的机制。
更新日期:2024-04-16
中文翻译:

具有不同水合位点的非异氰酸酯多羟基聚氨酯中水-聚合物相互作用和水驱动玻璃化转变降低的机制
研究了沿链具有不同仲氨基含量的非异氰酸酯聚氨酯(NIPU)的吸水性和增塑性。仲氨基促进水的吸收。根据各种吸附模型讨论了水活度范围 0-0.97 内的水吸附等温线。仲氨基充当额外的水合位点,增加单层容量。分配给氨基甲酸乙酯羰基的红外谱带红移,同时氨基甲酸乙酯 N-H 弯曲谱带蓝移,表明由于水分子和羟基氨基甲酸酯之间的强烈相互作用,聚合物-聚合物氢键在吸水时断裂。主要水合位点。随着水含量的增加,观察到玻璃化转变温度 () 降低。该效应是根据塑化和奴役机制进行讨论的,而在非常低的水合作用下的特殊下降归因于不遵循常见混合定律的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号