Structure ( IF 5.7 ) Pub Date : 2024-04-17 , DOI: 10.1016/j.str.2024.03.014 T. Bertie Ansell , Megan Healy , Claire E. Coupland , Mark S.P. Sansom , Christian Siebold
|
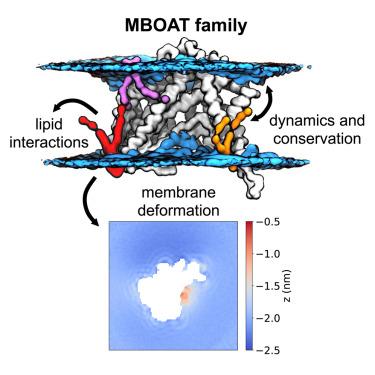
Membrane-bound O-acyltransferases (MBOATs) are membrane-embedded enzymes that catalyze acyl chain transfer to a diverse group of substrates, including lipids, small molecules, and proteins. MBOATs share a conserved structural core, despite wide-ranging functional specificity across both prokaryotes and eukaryotes. The structural basis of catalytic specificity, regulation and interactions with the surrounding environment remain uncertain. Here, we combine comparative molecular dynamics (MD) simulations with bioinformatics to assess molecular and interactional divergence across the family. In simulations, MBOATs differentially distort the bilayer depending on their substrate type. Additionally, we identify lipid binding sites surrounding reactant gates in the surrounding membrane. Complementary bioinformatic analyses reveal a conserved role for re-entrant loop-2 in MBOAT fold stabilization and a key hydrogen bond bridging DGAT1 dimerization. Finally, we predict differences in MBOAT solvation and water gating properties. These data are pertinent to the design of MBOAT-specific inhibitors that encompass dynamic information within cellular mimetic environments.
中文翻译:

绘制 MBOAT 系列的结构和动态差异
膜结合O -酰基转移酶 (MBOAT) 是膜嵌入酶,可催化酰基链转移至多种底物,包括脂质、小分子和蛋白质。尽管 MBOAT 在原核生物和真核生物中都具有广泛的功能特异性,但它们具有保守的结构核心。催化特异性、调节以及与周围环境相互作用的结构基础仍然不确定。在这里,我们将比较分子动力学(MD)模拟与生物信息学相结合,以评估整个家族的分子和相互作用差异。在模拟中,MBOAT 根据其基底类型对双层进行不同程度的扭曲。此外,我们还确定了周围膜中反应物门周围的脂质结合位点。补充生物信息学分析揭示了可重入环 2 在 MBOAT 折叠稳定中的保守作用以及桥接 DGAT1 二聚化的关键氢键。最后,我们预测了 MBOAT 溶剂化和水门控特性的差异。这些数据与 MBOAT 特异性抑制剂的设计相关,该抑制剂包含细胞模拟环境中的动态信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号