Structure ( IF 5.7 ) Pub Date : 2024-04-04 , DOI: 10.1016/j.str.2024.03.006 Tong Huo , Hongjiang Wu , Zeinab Moussa , Mehmet Sen , Valerie Dalton , Zhao Wang
|
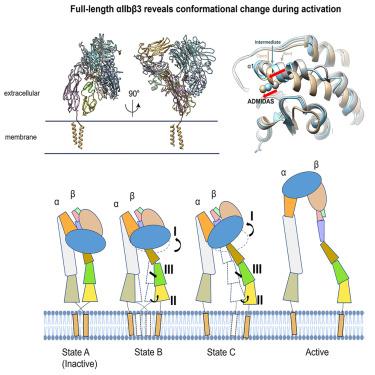
Integrin αIIbβ3 is the key receptor regulating platelet retraction and accumulation and a proven drug-target for antithrombotic therapies. Here we resolve the cryo-EM structures of the full-length αIIbβ3, which covers three distinct states along the activation pathway. Firstly, we obtain the αIIbβ3 structure at 3 Å resolution in the inactive state, revealing the overall topology of the heterodimer with the transmembrane (TM) helices and the ligand-binding domain tucked in a specific angle proximity to the TM region. After the addition of a Mn2+ agonist, we resolve two coexisting structures representing two new states between inactive and active state. Our structures show conformational changes of the αIIbβ3 activating trajectory and a unique twisting of the integrin legs, which is required for platelets accumulation. Our structure provides direct structural evidence for how the lower legs are involved in full-length integrin activation mechanisms and offers a new strategy to target the αIIbβ3 lower leg.
中文翻译:

全长 αIIbβ3 冷冻电镜结构揭示了完整的整合素起始激活内在结构
整合素 αIIbβ3 是调节血小板回缩和积聚的关键受体,也是抗血栓治疗的经过验证的药物靶点。在这里,我们解析了全长 αIIbβ3 的冷冻电镜结构,它涵盖了激活途径上的三个不同状态。首先,我们在非活性状态下获得了 3 Å 分辨率的 αIIbβ3 结构,揭示了异二聚体的整体拓扑结构,其中包括跨膜 (TM) 螺旋和以特定角度靠近 TM 区域的配体结合结构域。添加Mn 2+激动剂后,我们解决了代表非活性和活性状态之间的两种新状态的两个共存结构。我们的结构显示了 αIIbβ3 激活轨迹的构象变化和整合素腿的独特扭曲,这是血小板积累所必需的。我们的结构为小腿如何参与全长整合素激活机制提供了直接的结构证据,并提供了一种针对 αIIbβ3 小腿的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号