当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalytic Conversion of Methane to Ethanol at a Three-Phase Interface with Concentration-Matched Hydroxyl and Methyl Radicals
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.4c01366 Chun He 1 , Lan Shang 1 , Hongfu Zhu 1 , Lianchao Yu 1 , Lingzhi Wang 1 , Jinlong Zhang 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-04-17 , DOI: 10.1021/jacs.4c01366 Chun He 1 , Lan Shang 1 , Hongfu Zhu 1 , Lianchao Yu 1 , Lingzhi Wang 1 , Jinlong Zhang 1
Affiliation
|
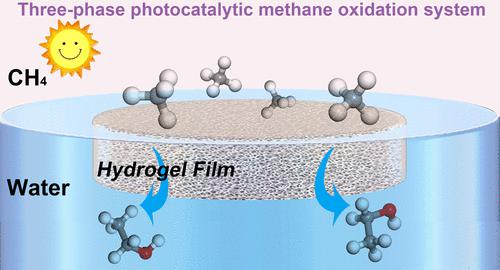
The direct oxidation of CH4 to C2H5OH is attractive but challenging owing to the intricate processes involving carbon-chain growth and hydroxylation simultaneously. The inherent difficulty arises from the strong tendency of CH4 to overoxidize in the commonly used pressurized powder suspension systems rich in reactive oxygen radicals (ROR), which are specifically designed for CH4 concentration and activation. Meanwhile, the strong tendency of nucleophilic attack of potent ROR on the C–C bond of the resulting product C2H5OH ultimately leads to a higher selectivity for C1 oxygenates. This study addresses this multifaceted issue by designing a three-phase interface based on a hydrophilic floating Fe(III)-cross-linked macroporous alginate hydrogel film encapsulated with C3N4 [Fe(III)@ACN] to simultaneously enhance the accessibility of H2O and CH4 molecules to the active sites and species within the macroporous channel. The hydrophilic properties of Fe(III)@ACN allow the in situ production of H2O2 from C3N4 through the water oxidation reaction under irradiation. The concurrent photoinduced Fe(II) triggers Fenton reaction with H2O2 to produce •OH. The enhanced mass transfer of CH4 at the three-phase interface ensures the efficient formation of •CH3 by reacting with •OH, ultimately facilitating carbon-chain growth in the conversion pathway from CH4 to CH3OH and finally to C2H5OH with •CH3 and •OH present in comparable concentrations. Thus, the Fe(III)@ACN catalyst exhibits a remarkable 96% selectivity for alcohol, achieving a 90% selectivity for C2H5OH in the alcohol products. The C2H5OH production rate reaches 171.7 μmol g–1 h–1 without the need for precious-metal additive.
中文翻译:

浓度匹配的羟基和甲基自由基在三相界面处将甲烷光催化转化为乙醇
CH 4直接氧化为C 2 H 5 OH 很有吸引力,但由于同时涉及碳链增长和羟基化的复杂过程而具有挑战性。固有的困难源于CH 4在常用的富含活性氧自由基(ROR)的加压粉末悬浮液系统中过度氧化的强烈倾向,该系统是专为CH 4浓缩和活化而设计的。同时,有效的ROR对所得产物C 2 H 5 OH的C-C键的强烈亲核攻击倾向最终导致对C1含氧化合物的更高选择性。本研究通过设计基于亲水性浮动 Fe(III) 交联大孔藻酸盐水凝胶膜的三相界面解决了这个多方面的问题,该膜被 C 3 N 4 [Fe(III)@ACN] 封装,同时增强了H 2 O和CH 4分子到达大孔通道内的活性位点和物质。 Fe(III)@ACN的亲水特性允许在辐射下通过水氧化反应由C 3 N 4原位产生H 2 O 2 。同时发生的光诱导 Fe(II) 引发与 H 2 O 2的 Fenton 反应,生成· OH。 CH 4在三相界面处增强的传质确保了通过与· OH 反应有效形成· CH 3 ,最终促进从CH 4到CH 3 OH并最终到C 2 H的转化途径中的碳链生长5 OH,其中• CH 3和• OH 以相当的浓度存在。因此,Fe(III)@ACN催化剂对醇表现出高达96%的选择性,对醇产物中的C 2 H 5 OH实现了90%的选择性。无需贵金属添加剂,C 2 H 5 OH产率可达171.7 μmol g –1 h –1 。
更新日期:2024-04-18
中文翻译:

浓度匹配的羟基和甲基自由基在三相界面处将甲烷光催化转化为乙醇
CH 4直接氧化为C 2 H 5 OH 很有吸引力,但由于同时涉及碳链增长和羟基化的复杂过程而具有挑战性。固有的困难源于CH 4在常用的富含活性氧自由基(ROR)的加压粉末悬浮液系统中过度氧化的强烈倾向,该系统是专为CH 4浓缩和活化而设计的。同时,有效的ROR对所得产物C 2 H 5 OH的C-C键的强烈亲核攻击倾向最终导致对C1含氧化合物的更高选择性。本研究通过设计基于亲水性浮动 Fe(III) 交联大孔藻酸盐水凝胶膜的三相界面解决了这个多方面的问题,该膜被 C 3 N 4 [Fe(III)@ACN] 封装,同时增强了H 2 O和CH 4分子到达大孔通道内的活性位点和物质。 Fe(III)@ACN的亲水特性允许在辐射下通过水氧化反应由C 3 N 4原位产生H 2 O 2 。同时发生的光诱导 Fe(II) 引发与 H 2 O 2的 Fenton 反应,生成· OH。 CH 4在三相界面处增强的传质确保了通过与· OH 反应有效形成· CH 3 ,最终促进从CH 4到CH 3 OH并最终到C 2 H的转化途径中的碳链生长5 OH,其中• CH 3和• OH 以相当的浓度存在。因此,Fe(III)@ACN催化剂对醇表现出高达96%的选择性,对醇产物中的C 2 H 5 OH实现了90%的选择性。无需贵金属添加剂,C 2 H 5 OH产率可达171.7 μmol g –1 h –1 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号