当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Synthesis of CoFe-Nanomesh for Oxygen Evolution Reaction
ACS Applied Nano Materials ( IF 5.9 ) Pub Date : 2024-04-17 , DOI: 10.1021/acsanm.3c05903 Shashank Sharma 1 , Amit Paul 1
ACS Applied Nano Materials ( IF 5.9 ) Pub Date : 2024-04-17 , DOI: 10.1021/acsanm.3c05903 Shashank Sharma 1 , Amit Paul 1
Affiliation
|
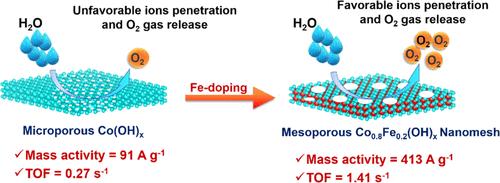
Iron-doped cobalt-based nanomeshes have been synthesized in a one-pot methodology. 2-Methyl imidazole (2-HMIM) acted as an etchant, while high valent iron increased the acidity of the solution and helped to synthesize ultrathin (thickness 1.9–2.0 nm) two-dimensional (2D)-nanomeshes having small uniform mesopores (3.8–4.0 nm) on the basal plane with high surface area and high pore volume. 20% iron-doped nanomesh material (Co0.8Fe0.2(OH)x) revealed the best water oxidation reactivity, having an overpotential of 314 ± 3 mV, a mass activity of 413 ± 19 A/g, a turnover frequency (TOF) of 1.41 ± 0.04 s–1, and a TOFEIS of 4.15 ± 0.04 s–1. Electrochemical results suggested that the Co0.8Fe0.2(OH)x-nanomesh had a double-layer capacitance of 12.2 mF/cm2, corresponding to a roughness factor of 452, i.e., ion accessibility inside the nanomaterial was improved 452 times compared to the geometrical surface area of the electrode. This remarkable reactivity was due to (a) improved active sites for water oxidation at protruding edge sites and narrow mesopores on the basal planes of nanomeshes, (b) narrow mesopores on the basal planes ensured vertical ion penetration, vacant sites for water oxidation, and easy O2 release from the material, and (c) electrochemical impedance spectroscopy results suggested that nanomesh formation ensured fast charge propagation inside the nanomaterial during the oxygen evolution reaction (OER) and fast charge transfer at the electrode/electrolyte interface.
中文翻译:

用于析氧反应的 CoFe-纳米网的一锅法合成
铁掺杂钴基纳米网已通过一锅法合成。 2-甲基咪唑(2-HMIM)充当蚀刻剂,而高价铁增加了溶液的酸度,有助于合成具有小均匀介孔(3.8)的超薄(厚度1.9-2.0 nm)二维(2D)纳米网。 –4.0 nm)在具有高表面积和高孔体积的基面上。 20%铁掺杂纳米网材料(Co 0.8 Fe 0.2 (OH) x)显示出最佳的水氧化反应性,过电势为314 ± 3 mV,质量活性为413 ± 19 A/g,周转频率(TOF) 1.41 ± 0.04 s –1,TOF EIS为 4.15 ± 0.04 s –1。电化学结果表明,Co 0.8 Fe 0.2 (OH) x纳米网的双层电容为12.2 mF/cm 2,对应的粗糙度因子为452,即纳米材料内部的离子可达性比普通纳米网提高了452倍。电极的几何表面积。这种显着的反应性是由于(a)纳米网基底平面上的突出边缘位置和狭窄介孔处的水氧化活性位点得到改善,(b)基底平面上的狭窄介孔确保了垂直离子渗透,水氧化的空位,以及O 2容易从材料中释放,(c)电化学阻抗谱结果表明,纳米网的形成确保了析氧反应(OER)过程中纳米材料内部的快速电荷传播以及电极/电解质界面处的快速电荷转移。
更新日期:2024-04-17
中文翻译:

用于析氧反应的 CoFe-纳米网的一锅法合成
铁掺杂钴基纳米网已通过一锅法合成。 2-甲基咪唑(2-HMIM)充当蚀刻剂,而高价铁增加了溶液的酸度,有助于合成具有小均匀介孔(3.8)的超薄(厚度1.9-2.0 nm)二维(2D)纳米网。 –4.0 nm)在具有高表面积和高孔体积的基面上。 20%铁掺杂纳米网材料(Co 0.8 Fe 0.2 (OH) x)显示出最佳的水氧化反应性,过电势为314 ± 3 mV,质量活性为413 ± 19 A/g,周转频率(TOF) 1.41 ± 0.04 s –1,TOF EIS为 4.15 ± 0.04 s –1。电化学结果表明,Co 0.8 Fe 0.2 (OH) x纳米网的双层电容为12.2 mF/cm 2,对应的粗糙度因子为452,即纳米材料内部的离子可达性比普通纳米网提高了452倍。电极的几何表面积。这种显着的反应性是由于(a)纳米网基底平面上的突出边缘位置和狭窄介孔处的水氧化活性位点得到改善,(b)基底平面上的狭窄介孔确保了垂直离子渗透,水氧化的空位,以及O 2容易从材料中释放,(c)电化学阻抗谱结果表明,纳米网的形成确保了析氧反应(OER)过程中纳米材料内部的快速电荷传播以及电极/电解质界面处的快速电荷转移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号