当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Photoenolization/Diels–Alder Reaction of 2-Methylbenzaldehydes and 2-Alkylbenzophenones with Chromones
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-16 , DOI: 10.1021/acscatal.4c01264 Yuhao Mo 1 , Lichao Ning 1 , Zhe Luo 1 , Liangkun Yang 1 , Shi Tang 1 , Shunxi Dong 1 , Qi-Lin Zhou 2 , Xiaoming Feng 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2024-04-16 , DOI: 10.1021/acscatal.4c01264 Yuhao Mo 1 , Lichao Ning 1 , Zhe Luo 1 , Liangkun Yang 1 , Shi Tang 1 , Shunxi Dong 1 , Qi-Lin Zhou 2 , Xiaoming Feng 1
Affiliation
|
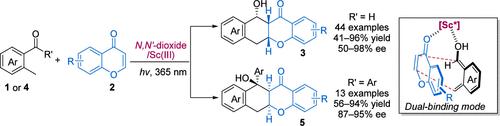
The asymmetric photoenolization/Diels–Alder reaction provides a straightforward and atom-economical route to complex chiral polycyclic rings. In comparison with well-developed transformations of 2-alkylbenzophenones, the enantioselective photoenolization/Diels–Alder reaction of 2-methylbenzaldehydes was challenging due to the shorter-lived and unstable photoenol intermediates. Herein, we present a highly enantioselective photoenolization/Diels–Alder reaction of 2-methylbenzaldehydes with chromones. Chiral N,N′-dioxide/ScIII and YbIII complexes were found to interact with both photoenol intermediates and chromones simultaneously, accelerating the Diels–Alder reaction in an efficient and stereoselective manner. Experimental studies and DFT calculations were carried out to understand the reaction mechanism and the origin of stereoselectivity. In addition, 2-alkylbenzophenones were suitable substrates. A series of chiral fused polycyclic rings with vicinal multisubstituted stereocenters were afforded in good yields and high diastereo- and enantioselectivities.
中文翻译:

2-甲基苯甲醛和2-烷基二苯甲酮与色酮的不对称光烯醇化/Diels-Alder反应
不对称光烯醇化/狄尔斯-阿尔德反应为复杂的手性多环提供了一种简单且原子经济的途径。与2-烷基二苯甲酮的成熟转化相比,2-甲基苯甲醛的对映选择性光烯醇化/Diels-Alder反应由于寿命较短且不稳定的光烯醇中间体而具有挑战性。在此,我们提出了 2-甲基苯甲醛与色酮的高度对映选择性光烯醇化/狄尔斯-阿尔德反应。发现手性N、N '-二氧化物/Sc III和 Yb III配合物同时与光烯醇中间体和色酮相互作用,以有效和立体选择性的方式加速 Diels-Alder 反应。通过实验研究和DFT计算来了解反应机理和立体选择性的起源。此外,2-烷基二苯甲酮也是合适的底物。以良好的产率和高的非对映选择性和对映选择性提供了一系列具有邻位多取代立体中心的手性稠合多环。
更新日期:2024-04-17
中文翻译:

2-甲基苯甲醛和2-烷基二苯甲酮与色酮的不对称光烯醇化/Diels-Alder反应
不对称光烯醇化/狄尔斯-阿尔德反应为复杂的手性多环提供了一种简单且原子经济的途径。与2-烷基二苯甲酮的成熟转化相比,2-甲基苯甲醛的对映选择性光烯醇化/Diels-Alder反应由于寿命较短且不稳定的光烯醇中间体而具有挑战性。在此,我们提出了 2-甲基苯甲醛与色酮的高度对映选择性光烯醇化/狄尔斯-阿尔德反应。发现手性N、N '-二氧化物/Sc III和 Yb III配合物同时与光烯醇中间体和色酮相互作用,以有效和立体选择性的方式加速 Diels-Alder 反应。通过实验研究和DFT计算来了解反应机理和立体选择性的起源。此外,2-烷基二苯甲酮也是合适的底物。以良好的产率和高的非对映选择性和对映选择性提供了一系列具有邻位多取代立体中心的手性稠合多环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号