当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
From scrap metal to highly efficient electrodes: harnessing the nanotextured surface of swarf for effective utilisation of Pt and Co for hydrogen production
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4ta00711e Madasamy Thangamuthu 1 , Emerson C. Kohlrausch 1 , Ming Li 2 , Alistair Speidel 2 , Adam T. Clare 2 , Richard Plummer 1 , Paul Geary 3 , James W. Murray 2 , Andrei N. Khlobystov 1 , Jesum Alves Fernandes 1
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2024-04-16 , DOI: 10.1039/d4ta00711e Madasamy Thangamuthu 1 , Emerson C. Kohlrausch 1 , Ming Li 2 , Alistair Speidel 2 , Adam T. Clare 2 , Richard Plummer 1 , Paul Geary 3 , James W. Murray 2 , Andrei N. Khlobystov 1 , Jesum Alves Fernandes 1
Affiliation
|
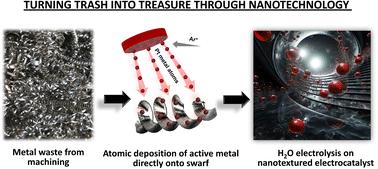
Hydrogen is considered to be the key element to achieving climate neutrality, leading to a massive demand for electrocatalysts. This work explores the transformation of metal waste into active and stable electrode materials for water splitting by modifying the surface through atomic deposition of platinum (Pt) and cobalt (Co). Our study finds that with the addition of only 28 μg cm−2 of Pt and 30 μg cm−2 of Co to metal waste, high-performance electrolysis can be achieved. We investigated discarded stainless-steel (SST), titanium (Ti), and nickel (Ni) alloys and found that they had nanotextured surfaces, consisting of 10–50 nm wide grooves, which offered an excellent platform for effective bonding of Pt or Co atoms. We demonstrate a strong synergistic relationship between the metal of the swarf surface and the metal of catalytically active centers, such that only some combinations lead to effective electrocatalysts. Furthermore, we discovered that the surface density of atomically deposited Pt or Co has a profound impact on the nanoscale morphology of the active centers, providing a mechanism for the optimization of electrocatalytic characteristics. For instance, the optimal Pt loading (28 μg cm−2) on Ti swarf yields 5–20 nm Pt nanoparticles within the grooves with exceptional hydrogen evolution reaction (HER) activity. Similarly, the optimal surface density of Co (30 μg cm−2) on Ni swarf generates ∼100 nm interlinked flakes of Co(OH)2 with outstanding oxygen evolution reaction (OER) performance. Combining these best electrodes in a full-cell electrolyser resulted in a current density of 40 mA cm−2 at 1.6 V vs. RHE and the rates of H2 and O2 production of 22.09 and 10.75 mmol min−1, respectively, with 100% faradaic efficiency with no decrease in activity in 24 hours. This study opens the door to more sustainable electrode fabrication and effective hydrogen production in alkaline water electrolysis.
中文翻译:

从废金属到高效电极:利用金属屑的纳米纹理表面有效利用 Pt 和 Co 来生产氢气
氢被认为是实现气候中和的关键元素,导致对电催化剂的巨大需求。这项工作探索了通过铂(Pt)和钴(Co)的原子沉积对表面进行改性,将金属废料转化为活性稳定的水分解电极材料。我们的研究发现,在金属废料中仅添加28 μg cm -2的Pt和30 μg cm -2的Co,就可以实现高性能电解。我们研究了废弃的不锈钢 (SST)、钛 (Ti) 和镍 (Ni) 合金,发现它们具有纳米纹理表面,由 10-50 nm 宽的凹槽组成,这为 Pt 或 Co 的有效结合提供了极好的平台原子。我们证明了切屑表面的金属和催化活性中心的金属之间存在很强的协同关系,因此只有某些组合才能产生有效的电催化剂。此外,我们发现原子沉积的 Pt 或 Co 的表面密度对活性中心的纳米级形貌具有深远的影响,为优化电催化特性提供了机制。例如,Ti 切屑上的最佳 Pt 负载量 (28 μg cm -2 ) 会在凹槽内产生具有出色析氢反应 (HER) 活性的 5–20 nm Pt 纳米颗粒。类似地, Ni 切屑上Co (30 μg cm -2 )的最佳表面密度可生成约 100 nm 的 Co(OH) 2互连薄片,具有出色的析氧反应 (OER) 性能。将这些最佳电极组合在全电池电解槽中,在 1.6 V vs. RHE 下产生 40 mA cm -2的电流密度,并且在 100 条件下,H 2和 O 2的生成速率分别为 22.09 和 10.75 mmol min -1 。 % 法拉第效率,24 小时内活性没有下降。这项研究为碱性水电解中更可持续的电极制造和有效的氢气生产打开了大门。
更新日期:2024-04-16
中文翻译:

从废金属到高效电极:利用金属屑的纳米纹理表面有效利用 Pt 和 Co 来生产氢气
氢被认为是实现气候中和的关键元素,导致对电催化剂的巨大需求。这项工作探索了通过铂(Pt)和钴(Co)的原子沉积对表面进行改性,将金属废料转化为活性稳定的水分解电极材料。我们的研究发现,在金属废料中仅添加28 μg cm -2的Pt和30 μg cm -2的Co,就可以实现高性能电解。我们研究了废弃的不锈钢 (SST)、钛 (Ti) 和镍 (Ni) 合金,发现它们具有纳米纹理表面,由 10-50 nm 宽的凹槽组成,这为 Pt 或 Co 的有效结合提供了极好的平台原子。我们证明了切屑表面的金属和催化活性中心的金属之间存在很强的协同关系,因此只有某些组合才能产生有效的电催化剂。此外,我们发现原子沉积的 Pt 或 Co 的表面密度对活性中心的纳米级形貌具有深远的影响,为优化电催化特性提供了机制。例如,Ti 切屑上的最佳 Pt 负载量 (28 μg cm -2 ) 会在凹槽内产生具有出色析氢反应 (HER) 活性的 5–20 nm Pt 纳米颗粒。类似地, Ni 切屑上Co (30 μg cm -2 )的最佳表面密度可生成约 100 nm 的 Co(OH) 2互连薄片,具有出色的析氧反应 (OER) 性能。将这些最佳电极组合在全电池电解槽中,在 1.6 V vs. RHE 下产生 40 mA cm -2的电流密度,并且在 100 条件下,H 2和 O 2的生成速率分别为 22.09 和 10.75 mmol min -1 。 % 法拉第效率,24 小时内活性没有下降。这项研究为碱性水电解中更可持续的电极制造和有效的氢气生产打开了大门。



























 京公网安备 11010802027423号
京公网安备 11010802027423号