Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Superparamagnetic Iron Oxide Nanoparticles Reprogram the Tumor Microenvironment and Reduce Lung Cancer Regrowth after Crizotinib Treatment
ACS Nano ( IF 17.1 ) Pub Date : 2024-04-16 , DOI: 10.1021/acsnano.3c08335 Natalie K. Horvat 1, 2, 3 , Sara Chocarro 3, 4 , Oriana Marques 1, 2 , Tobias A. Bauer 5 , Ruiyue Qiu 1 , Alberto Diaz-Jimenez 3, 4 , Barbara Helm 6, 7 , Yuanyuan Chen 4 , Stefan Sawall 8 , Richard Sparla 1 , Lu Su 5 , Ursula Klingmüller 6, 7, 9 , Matthias Barz 5, 10 , Matthias W. Hentze 2, 11 , Rocío Sotillo 4, 7, 9 , Martina U. Muckenthaler 1, 2, 7, 12
ACS Nano ( IF 17.1 ) Pub Date : 2024-04-16 , DOI: 10.1021/acsnano.3c08335 Natalie K. Horvat 1, 2, 3 , Sara Chocarro 3, 4 , Oriana Marques 1, 2 , Tobias A. Bauer 5 , Ruiyue Qiu 1 , Alberto Diaz-Jimenez 3, 4 , Barbara Helm 6, 7 , Yuanyuan Chen 4 , Stefan Sawall 8 , Richard Sparla 1 , Lu Su 5 , Ursula Klingmüller 6, 7, 9 , Matthias Barz 5, 10 , Matthias W. Hentze 2, 11 , Rocío Sotillo 4, 7, 9 , Martina U. Muckenthaler 1, 2, 7, 12
Affiliation
|
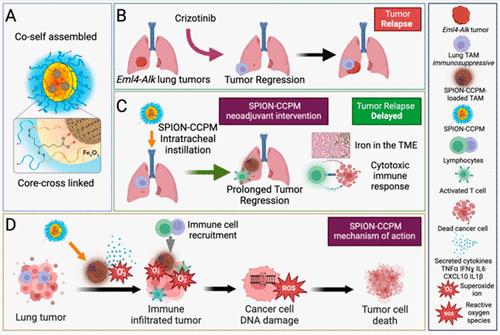
ALK-positive NSCLC patients demonstrate initial responses to ALK tyrosine kinase inhibitor (TKI) treatments, but eventually develop resistance, causing rapid tumor relapse and poor survival rates. Growing evidence suggests that the combination of drug and immune therapies greatly improves patient survival; however, due to the low immunogenicity of the tumors, ALK-positive patients do not respond to currently available immunotherapies. Tumor-associated macrophages (TAMs) play a crucial role in facilitating lung cancer growth by suppressing tumoricidal immune activation and absorbing chemotherapeutics. However, they can also be programmed toward a pro-inflammatory tumor suppressive phenotype, which represents a highly active area of therapy development. Iron loading of TAMs can achieve such reprogramming correlating with an improved prognosis in lung cancer patients. We previously showed that superparamagnetic iron oxide nanoparticles containing core-cross-linked polymer micelles (SPION-CCPMs) target macrophages and stimulate pro-inflammatory activation. Here, we show that SPION-CCPMs stimulate TAMs to secrete reactive nitrogen species and cytokines that exert tumoricidal activity. We further show that SPION-CCPMs reshape the immunosuppressive Eml4-Alk lung tumor microenvironment (TME) toward a cytotoxic profile hallmarked by the recruitment of CD8+ T cells, suggesting a multifactorial benefit of SPION-CCPM application. When intratracheally instilled into lung cancer-bearing mice, SPION-CCPMs delay tumor growth and, after first line therapy with a TKI, halt the regrowth of relapsing tumors. These findings identify SPIONs-CCPMs as an adjuvant therapy, which remodels the TME, resulting in a delay in the appearance of resistant tumors.
中文翻译:

超顺磁性氧化铁纳米颗粒重新编程肿瘤微环境并减少克唑替尼治疗后肺癌的再生
ALK 阳性 NSCLC 患者对 ALK 酪氨酸激酶抑制剂 (TKI) 治疗表现出初步反应,但最终产生耐药性,导致肿瘤快速复发和生存率低下。越来越多的证据表明,药物和免疫疗法的结合可以大大提高患者的生存率。然而,由于肿瘤的免疫原性较低,ALK 阳性患者对目前可用的免疫疗法没有反应。肿瘤相关巨噬细胞(TAM)通过抑制杀肿瘤免疫激活和吸收化疗药物,在促进肺癌生长方面发挥着至关重要的作用。然而,它们也可以被编程为促炎性肿瘤抑制表型,这代表了治疗开发的一个高度活跃的领域。 TAM 的铁负载可以实现这种重编程,从而改善肺癌患者的预后。我们之前表明,含有核心交联聚合物胶束(SPION-CCPM)的超顺磁性氧化铁纳米颗粒可靶向巨噬细胞并刺激促炎激活。在这里,我们表明 SPION-CCPM 刺激 TAM 分泌活性氮和细胞因子,发挥杀肿瘤活性。我们进一步表明,SPION-CCPM 重塑了免疫抑制性Eml4-Alk肺肿瘤微环境 (TME),使其趋向以招募 CD8 + T 细胞为标志的细胞毒性特征,这表明 SPION-CCPM 应用具有多因素益处。当气管内注入患有肺癌的小鼠时,SPION-CCPM 可以延迟肿瘤生长,并且在使用 TKI 进行一线治疗后,可以阻止复发肿瘤的再生长。这些发现将 SPIONs-CCPMs 确定为一种辅助疗法,它可以重塑 TME,从而延迟耐药肿瘤的出现。
更新日期:2024-04-16
中文翻译:

超顺磁性氧化铁纳米颗粒重新编程肿瘤微环境并减少克唑替尼治疗后肺癌的再生
ALK 阳性 NSCLC 患者对 ALK 酪氨酸激酶抑制剂 (TKI) 治疗表现出初步反应,但最终产生耐药性,导致肿瘤快速复发和生存率低下。越来越多的证据表明,药物和免疫疗法的结合可以大大提高患者的生存率。然而,由于肿瘤的免疫原性较低,ALK 阳性患者对目前可用的免疫疗法没有反应。肿瘤相关巨噬细胞(TAM)通过抑制杀肿瘤免疫激活和吸收化疗药物,在促进肺癌生长方面发挥着至关重要的作用。然而,它们也可以被编程为促炎性肿瘤抑制表型,这代表了治疗开发的一个高度活跃的领域。 TAM 的铁负载可以实现这种重编程,从而改善肺癌患者的预后。我们之前表明,含有核心交联聚合物胶束(SPION-CCPM)的超顺磁性氧化铁纳米颗粒可靶向巨噬细胞并刺激促炎激活。在这里,我们表明 SPION-CCPM 刺激 TAM 分泌活性氮和细胞因子,发挥杀肿瘤活性。我们进一步表明,SPION-CCPM 重塑了免疫抑制性Eml4-Alk肺肿瘤微环境 (TME),使其趋向以招募 CD8 + T 细胞为标志的细胞毒性特征,这表明 SPION-CCPM 应用具有多因素益处。当气管内注入患有肺癌的小鼠时,SPION-CCPM 可以延迟肿瘤生长,并且在使用 TKI 进行一线治疗后,可以阻止复发肿瘤的再生长。这些发现将 SPIONs-CCPMs 确定为一种辅助疗法,它可以重塑 TME,从而延迟耐药肿瘤的出现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号