当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Tyrosine Amination: Detection, Imaging, and Chemoproteomic Profiling with Synthetic Probes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-15 , DOI: 10.1021/jacs.4c01028 Lei Chen 1 , Tonghua Yang 1 , Xue Sun 2 , Catherine C L Wong 2, 3, 4 , Dan Yang 5, 6
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-04-15 , DOI: 10.1021/jacs.4c01028 Lei Chen 1 , Tonghua Yang 1 , Xue Sun 2 , Catherine C L Wong 2, 3, 4 , Dan Yang 5, 6
Affiliation
|
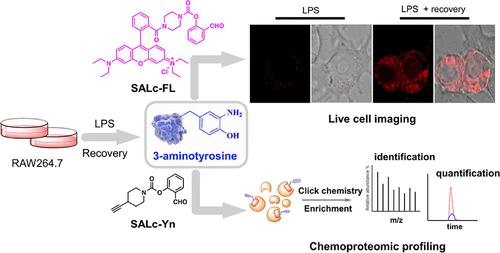
Protein tyrosine nitration (PTN) by oxidative and nitrative stress is a well-known post-translational modification that plays a role in the initiation and progression of various diseases. Despite being recognized as a stable modification for decades, recent studies have suggested the existence of a reduction in PTN, leading to the formation of 3-aminotyrosine (3AT) and potential denitration processes. However, the vital functions of 3AT-containing proteins are still unclear due to the lack of selective probes that directly target the protein tyrosine amination. Here, we report a novel approach to label and enrich 3AT-containing proteins with synthetic salicylaldehyde (SAL)-based probes: SALc-FL with a fluorophore and SALc-Yn with an alkyne tag. These probes exhibit high selectivity and efficiency in labeling and can be used in cell lysates and live cells. More importantly, SALc-Yn offers versatility when integrated into multiple platforms by enabling proteome-wide quantitative profiling of cell nitration dynamics. Using SALc-Yn, 355 proteins were labeled, enriched, and identified to carry the 3AT modification in oxidatively stressed RAW264.7 cells. These findings provide compelling evidence supporting the involvement of 3AT as a critical intermediate in nitrated protein turnover. Moreover, our probes serve as powerful tools to investigate protein nitration and denitration processes, and the identification of 3AT-containing proteins contributes to our understanding of PTN dynamics and its implications in cellular redox biology.
中文翻译:

蛋白质酪氨酸胺化:使用合成探针进行检测、成像和化学蛋白质组学分析
氧化和硝化应激引起的蛋白质酪氨酸硝化(PTN)是一种众所周知的翻译后修饰,在多种疾病的发生和进展中发挥作用。尽管数十年来一直被认为是一种稳定的修饰,但最近的研究表明 PTN 的存在减少,导致 3-氨基酪氨酸 (3AT) 的形成和潜在的脱硝过程。然而,由于缺乏直接靶向蛋白质酪氨酸氨基化的选择性探针,含3AT的蛋白质的重要功能仍不清楚。在这里,我们报告了一种使用基于合成水杨醛 (SAL) 的探针标记和富集含 3AT 的蛋白质的新方法:带有荧光团的SALc-FL和带有炔标签的SALc-Yn 。这些探针在标记方面表现出高选择性和效率,可用于细胞裂解物和活细胞。更重要的是, SALc-Yn在集成到多个平台时可实现细胞硝化动力学的蛋白质组范围内的定量分析,从而提供多功能性。使用SALc-Yn标记、富集并鉴定出 355 种蛋白质在氧化应激的 RAW264.7 细胞中携带 3AT 修饰。这些发现提供了令人信服的证据,支持 3AT 作为硝化蛋白质周转的关键中间体。此外,我们的探针可以作为研究蛋白质硝化和脱硝过程的强大工具,并且含 3AT 的蛋白质的鉴定有助于我们了解 PTN 动力学及其在细胞氧化还原生物学中的影响。
更新日期:2024-04-15
中文翻译:

蛋白质酪氨酸胺化:使用合成探针进行检测、成像和化学蛋白质组学分析
氧化和硝化应激引起的蛋白质酪氨酸硝化(PTN)是一种众所周知的翻译后修饰,在多种疾病的发生和进展中发挥作用。尽管数十年来一直被认为是一种稳定的修饰,但最近的研究表明 PTN 的存在减少,导致 3-氨基酪氨酸 (3AT) 的形成和潜在的脱硝过程。然而,由于缺乏直接靶向蛋白质酪氨酸氨基化的选择性探针,含3AT的蛋白质的重要功能仍不清楚。在这里,我们报告了一种使用基于合成水杨醛 (SAL) 的探针标记和富集含 3AT 的蛋白质的新方法:带有荧光团的SALc-FL和带有炔标签的SALc-Yn 。这些探针在标记方面表现出高选择性和效率,可用于细胞裂解物和活细胞。更重要的是, SALc-Yn在集成到多个平台时可实现细胞硝化动力学的蛋白质组范围内的定量分析,从而提供多功能性。使用SALc-Yn标记、富集并鉴定出 355 种蛋白质在氧化应激的 RAW264.7 细胞中携带 3AT 修饰。这些发现提供了令人信服的证据,支持 3AT 作为硝化蛋白质周转的关键中间体。此外,我们的探针可以作为研究蛋白质硝化和脱硝过程的强大工具,并且含 3AT 的蛋白质的鉴定有助于我们了解 PTN 动力学及其在细胞氧化还原生物学中的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号